| Chemical Properties | 2-Tridecanone has a warm, oily, herbaceous odor reminiscent of nut. |
| Chemical Properties | white to slightly yellow crystalline solid |
| Occurrence | Reported found in coconut and palm oils; also in the oil of Schizandra nigra Max. (Matsubusa). Also reported found in American cranberry, rabbiteye blueberry, raspberry, other types of ginger, blue cheeses, cheddar cheese, Swiss cheese, Camembert cheese, Gruyere cheese, Limburger cheese, parmesan cheese, other cheeses, grapefruit juice, fejoia fruit, onion, shallot, leek, chive, ginger, butter, milk, cream, milk powder, roast chicken, chicken fat, cooked beef and mutton, pork liver, hop oil, cognac, rum, coconut meat, mango, rice, corn oil, wort, dried bonito, mountain papaya and maté. |
| Uses | 2-Tridecanone was used to study the impact of soil amendments in onion bulbs. |
| Preparation | By heating a mixture of lauric acid and acetic acid over thorium oxide at 450°C. |
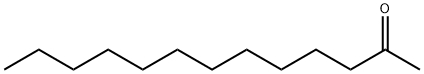
| Definition | ChEBI: A methyl ketone that is tridecane in which the methylene hydrogens at position 2 are replaced by an oxo group. |
| Taste threshold values | Taste characteristics at 10 ppm: fatty and earthy with a fatty mouthfeel and with a dairy, ketonic, waxy, creamy, cheesy, dairy and coconut. |
| General Description | White crystalline solid. |
| Air & Water Reactions | Insoluble in water. |
| Reactivity Profile | Ketones, such as 2-Tridecanone, are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4. |
| Fire Hazard | 2-Tridecanone is combustible. |