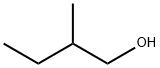
| Chemical Properties | Amyl alcohols (pentanols) have eight isomers. All are flammable, colorless liquids, except the isomer 2,2- dimethyl-1-propanol, which is a crystalline solid. |
| Chemical Properties | clear colorless to very slighlty yellow liquid |
| Chemical Properties | (+/–)2-Methyl-1-butanol has a cooked, roasted aroma with fruity or alcoholic undernotes. |
| Occurrence | Reportedly present in over 120 natural food products, including apple, apricot, banana, orange, bilberry, black currant, cranberry, papaya, strawberry, tomato and alcoholic beverages |
| Uses | 2-Methyl-1-butanol is a whiskey-scented amyl alcohol that is naturally present in all fruits wine and beer. 2-Methyl-1-butanol is commercially used as a solvent in paints and oils and as flavorant in many processed foods. 2-Methyl-1-butanol has a characteristic redolence, which is thought to account for its active properties as an attractant for hornets and certain wasps, such as yellowjackets, in traps. As a biochemical active ingredient, it has a non-toxic mode of action – targeted pests are killed through physical entrapment. |
| Uses | 2-Methyl-1-butanol is a volatile metabolite produced by a number of different plant species. Used as a solvent in organic synthesis (introduction of active amyl group), in lubricants, plasticizers, additives for oils & paints. It is also employed as a perfuming agent. |
| Uses | Solvent, organic synthesis (introduction of active amyl group), lubricants, plasticizers, additives for oils and paints. |
| Definition | The active alcohol from fusel oil. The synthetic product is a racemic mixture of both dextroand levorotatory compounds and therefore not optically active. |
| Production Methods | 2-methyl-1-butanol are refined from ethanol production as fusel oil. Isoamyl alcohols are used as solvents for oils, fats, resins, and waxes; in the plastics industry in spinning polyacrylonitrile; and in manufacturing lacquers, chemicals, and pharmaceuticals. |
| Preparation | Prepared from hydroboration of 2-methyl-1-butene. (–)2-Methyl-1-butanol is isolated by fractional distillation of fusel oil |
| Aroma threshold values | Odor threshold in air: detection at 0.14 mg/m3; recognition at 0.83 to 1.7 mg/m3. |
| General Description | S-(-)-2-Methyl-1-butanol is a precursor for the synthesis of chiral liquid crystals. It is a potential new-biofuel. |
| Hazard | Moderate fire and explosion risk. Toxic by ingestion, inhalation, and skin absorption. |
| Flammability and Explosibility | Flammable |
| Safety Profile | Moderately toxic by skin contact and intraperitoneal routes. Mddly toxic by ingestion. An eye, skin, and mucous membrane irritant. Can cause deafness, delirium, headache, nausea, and vomiting. Flammable liquid when exposed to heat, flame, or oxidizers. Explosive in the form of vapor when exposed to heat or flame. Incompatible with H2S3. To fight fire, use alcohol foam, spray, mist, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALCOHOLS. |
| Potential Exposure | (n-isomer); Suspected reprotoxic hazard, Primary irritant (w/o allergic reaction), (iso-, primary): Possible risk of forming tumors, Primary irritant (w/o allergic reaction), (sec-, active primary-, and other isomers) Primary irritant (w/o allergic reaction). Used as a solvent in organic synthesis and synthetic flavoring, pharmaceuticals, corrosion inhibitors; making plastics and other chemicals; as a flotation agent. The (n-isomer) is used in preparation of oil additives, plasticizers, synthetic lubricants, and as a solvent. |
| Shipping | UN2811 Pentanols, Hazard Class: 3; Labels: 3- Flammable liquid. UN1987 Alcohols, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid. |
| Purification Methods | Reflux the butanol with CaO, distil, reflux with magnesium and again fractionally distil it. A small sample of highly purified material is obtained by fractional crystallisation after conversion into a suitable ester such as the trinitrophthalate or the 3-nitrophthalate. The latter is converted to the cinchonine salt in acetone and recrystallised from CHCl3 by adding pentane. The salt is saponified, extracted with ether, and fractionally distilled. [Terry et al. J Chem Eng Data 5 403 1960, Beilstein 1 IV 1666.] |
| Incompatibilities | Forms an explosive mixture with air. Contact with strong oxidizers and hydrogen trisulfide may cause fire and explosions. Incompatible with strong acids. Violent reaction with alkaline earth metals forming hydrogen, a flammable gas. |
| Waste Disposal | Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. |