| Cadmium nitrate Basic information |
| Product Name: | Cadmium nitrate |
| Synonyms: | CADMIUM NITRATE;Cadmium standard for AAS;Cadmium, certified metal standard solution for atomic spectrometry;Nitricacid,cadmiumsalt;ai3-51840;CADMIUM ION STANDARD SOLUTION FLUKA, FOR ISE;CADMIUM ICP STANDARD SOLUTION FLUKA FOR;CADMIUM ION CHROMATOGRAPHY STD SOL. FLUK A, IN NITRIC AC. |
| CAS: | 10325-94-7 |
| MF: | CdN2O6 |
| MW: | 236.42 |
| EINECS: | 233-710-6 |
| Product Categories: | Extractable Heavy metalsSpectroscopy;Inorganics;Alphabetic;2000/60/ECMore…Close…;AAS;AAS CRMsAlphabetic;AASSpectroscopy;Application CRMs;C;CA – CGMethod Specific;European Community: ISO and DIN;Matrix Selection;NitrateMethod Specific;Oeko-Tex Standard 100;Spectroscopy;CA – CGChromatography;Cationic Standard Solutions;Ion Chromatography Standards |
| Mol File: | 10325-94-7.mol |
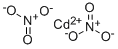 |
| Cadmium nitrate Chemical Properties |
| Melting point | 59.4℃ |
| Boiling point | 132℃ |
| density | 2.455(17/4℃) |
| form | white cubic crystals |
| PH | ≥3 |
| Water Solubility | g/100g solution H2O: 55.1 (0.6°C), 61.3 (25°C), 87.2 (100°C); solid phase, Cd(NO3)2 · 4H2O (0°C, 25°C), Cd(NO3)2 (100°C) [KRU93]; soluble alcohol, ammonia [HAW93] |
| Merck | 13,1622 |
| BRN | 8137359 |
| CAS DataBase Reference | 10325-94-7(CAS DataBase Reference) |
| EPA Substance Registry System | Cadmium nitrate (10325-94-7) |
| Safety Information |
| Hazard Codes | T,N,Xn |
| Risk Statements | 45-36/38-20/21/22-51/53-50/53-48/20/22-23-22-61-60-46-20/22 |
| Safety Statements | 53-45-36-26-36/37-60-61 |
| RIDADR | UN 3082 9/PG 3 |
| WGK Germany | 3 |
| HazardClass | 6.1(b) |
| PackingGroup | II |
| Hazardous Substances Data | 10325-94-7(Hazardous Substances Data) |
| Cadmium nitrate Usage And Synthesis |
| Chemical Properties | White, amorphous pieces or hygro- scopic needles. Soluble in water, ammonia, and alco- hol. |
| Physical properties | White crystal or amorphous powder; hygroscopic; density 3.60 g/cm3; melts at 350°C; very soluble in water, also soluble in alcohols. |
| Uses | Cadmium salts, photographic emulsions, col- oring glass and porcelain, laboratory reagent, cad- mium salts. |
| Definition | ChEBI: An inorganic nitrate salt having Cd(2+) as the counterion. |
| Preparation | reparation Cadmium nitrate is prepared by dissolving cadmium metal or its oxide, hydroxide, or carbonate, in nitric acid followed by crystallization: CdO + 2HNO3 → Cd(NO3)2 + H2O. |
| General Description | Odorless white solid. Sinks in water. |
| Air & Water Reactions | Water soluble. |
| Reactivity Profile | Mixtures of metal/nonmetal nitrates with alkyl esters may explode, owing to the formation of alkyl nitrates; mixtures a nitrate with phosphorus, tin (II) chloride, or other reducing agents may react explosively [Bretherick 1979 p. 108-109]. |
| Hazard | Dangerous fire and explosion hazard. |
| Health Hazard | Inhalation of fumes can produce coughing, chest constriction, headache, nausea, vomiting, pneumonitis. Chronic poisoning is characterized by emphysema and kidney injury. Ingestion causes gastrointestinal disturbance and severe toxic symptoms; both kidney and liver injuries may occur. Contact with eyes causes irritation. |
| Safety Profile | Confirmed human carcinogen. Poison by ingestion and possibly other routes. Moderately toxic by inhalation. Mutation data reported. When heated to decomposition it emits very toxic fumes of Cd and NOx. See also CADMIUM COMPOUNDS and NITRATES. |
| Cadmium nitrate Preparation Products And Raw materials |
| Raw materials | Nitric acid–>CADMIUM |
| Preparation Products | Cadmium acetate dihydrate |