| Chemical Properties | Colorless liquid with a foul odor. Insoluble in water, easily soluble in ethanol and ether. |
| Uses | Isobutyronitrile can be derived from isobutyraldehyde. It is used in organic synthesis, as a catalyst in the polymerization of ethylene and in the petroleum industry as a gasoline additive. Isobutyronitrile is also used to synthesize the intermediate 2-isopropyl-4-methyl-6-hydroxypyrimidine of the organophosphorus insecticide diazinon. |
| Production Methods | Isobutyronitrile is prepared from isobutyraldehyde by cyanation with ammonia. |
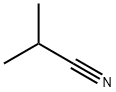
| Definition | ChEBI: Isobutyronitrile is an aliphatic nitrile that is acetonitrile in which two of the hydrogens have been replaced by methyl groups. It has a role as a polar aprotic solvent. It is an aliphatic nitrile and a volatile organic compound. |
| General Description | A clear colorless liquid. Flash point 47°F. Less dense than water. Vapors heavier than air. Toxic oxides of nitrogen produced during combustion. |
| Air & Water Reactions | Highly flammable. Slightly soluble in water. |
| Reactivity Profile | Isobutyronitrile is incompatible with the following: Oxidizers, reducing agents, strong acids & bases . |
| Hazard | Toxic by ingestion, inhalation, and skin absorption. |
| Health Hazard | Isobutyronitrile is considered highly hazardous and full precautions should be taken to prevent skin contact or inhalation of vapor. Inhaled isobutyronitrile is about 2.4 times as toxic as acetonitrile in rats. may be fatal if inhaled, swallowed, or absorbed through skin. Contact may cause burns to skin and eyes. (Non-Specific — Nitriles) Primarily, they are skin and eye irritants. Large doses cause collapse and stop breathing. In order to protect workers, the recommended TWA limit is obtained by dividing that for acetonitrile by the factor 2.4. NIOSH has therefore recommended that employee exposure should not exceed 8 p.p.m. (22 mg/m3) for either compound as a TLV-TWA. |
| Fire Hazard | Vapor may explode if ignited in an enclosed area. Toxic oxides of nitrogen are produced during combustion. Isobutyronitrile is a flammable/combustible material and may be ignited by heat, sparks, or flames. Vapors may travel to a source of ignition and flash back. Container may explode in heat of fire. Vapor explosion and poison hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Hazardous polymerization may not occur. |
| Metabolism | Thiocyanate was present in the urine of rats dosed orally with isobutyronitrile. |
| Purification Methods | Shake the nitrile with conc HCl (to remove isonitriles), then with water and aqueous NaHCO3. After a preliminary drying with silica gel or Linde type 4A molecular sieves, it is shaken or stirred with CaH2 until hydrogen evolution ceases, then decanted and distilled from P2O5 (not more than 5g/L, to minimize gel formation) or Drierite (b 101-103o/760mm). Finally it is refluxed with, and slowly distilled from CaH2 (5g/L), taking precautions to exclude moisture. [Beilstein 2 H 294, 2 I 129, 2 II 263, 2 III 655, 2 IV 853.] |