| Product Name: | (-)-Epicatechin gallate |
| Synonyms: | EPICATECHIN GALLATE, (-)- SNAP-N-SHOOT 0.1mg/ml(P);EPICATECHIN GALLATE, (-)-(P);8-methoxy-12a-methyl-3,4,4a,4b,5,6,10b,11,12,12a-decahydrochrysen-2(1h)-one;epicatechol,gallate;l-epicatechingallate;EPICATECHIN GALLATE(ECG) FROM GREEN TEA 50% BY HPLC;EPICATECHIN GALLATE(ECG) FROM GREEN TEA 70% BY HPLC;(-)-ECG:BENZOICACID, 3,4,5- TRIHYDROXY-, 2-(3,4-DIHYDROXYPHENYL)-3,4-DIHYDRO-5,7-DIHYDROXY-2H-1-BENZOPYRAN-3-YLESTER, (2R-CIS)-, |
| CAS: | 1257-08-5 |
| MF: | C22H18O10 |
| MW: | 442.37 |
| EINECS: | 603-088-4 |
| Product Categories: | Pharmaceutical Raw Materials;Inhibitors;chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;Aromatics;Chiral Reagents;Intermediates & Fine Chemicals;Pharmaceuticals;ECG |
| Mol File: | 1257-08-5.mol |
 |
| (-)-Epicatechin gallate Chemical Properties |
| Melting point | 257-258°C |
| alpha | -182~-194°(D/20℃)(c=0.2,CH3OH) |
| Boiling point | 861.7±65.0 °C(Predicted) |
| density | 1.80±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Acetone (Sparingly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 7.75±0.25(Predicted) |
| color | White to Off-White |
| Water Solubility | Soluble in water, acetone, DMSO, methanol. |
| Stability: | Light Sensitive |
| InChIKey | LSHVYAFMTMFKBA-TZIWHRDSSA-N |
| LogP | 2.670 (est) |
| CAS DataBase Reference | 1257-08-5(CAS DataBase Reference) |
| Safety Information |
| Provider | Language |
|---|---|
| SigmaAldrich | English |
| (-)-Epicatechin gallate Usage And Synthesis |
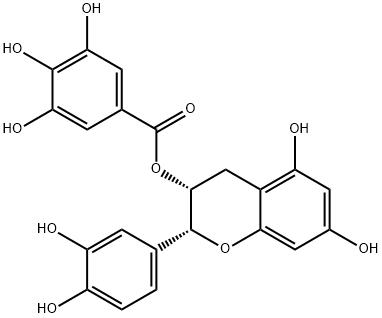
| Active ingredients of the Tea Polyphenol | Epicatechin gallate briefly referred to as EGCG, being the ester formed by epigallocatechin and gallate acid, belonging to a kind of tea polyphenols; is a kind of flavonoids, D-form ester catechins. Epicatechin gallate is the cathchin monomer isolated from the tea, being the major active ingredients of polyphenols.  Figure 1 shows the stereochemical structure of epicatechin gallate |
| Tea polyphenols | Tea polyphenols are the general term of the tea polyphenols, being a kind of polyphenols using catechins as the major body with biological oxidation effect. Chinese tea usually contains 20% to 30% tea polyphenols. Tea polyphenols can be divided into five categories, of which the flavanol (mainly catechins) is the most important; besides there are also anthocyanins, flavonoids, flavonols and phenolic acids. Catechins account for about 50% to 70% of the total amount of tea polyphenols, accounting for 12% to 24% of the dry weight of tea. It is a compound of complex structure, including four kinds of simple catechins (also called non-esters type catechins) and two kinds of complex catechins (also known as ester type catechins). The trend of the content of tea polyphenols is generally as below: green tea is higher than black tea; summer and autumn tea are more than spring tea. The main pharmacological effects of tea polyphenols are: (1) Lower the blood lipid, inhibit atherosclerosis. (2) Enhance the capillaries, lower blood sugar. (3) Anti-radiation. (4) Anti-aging. (5) Anti-cancer and anti-mutation. (6) Bactericidal effect. 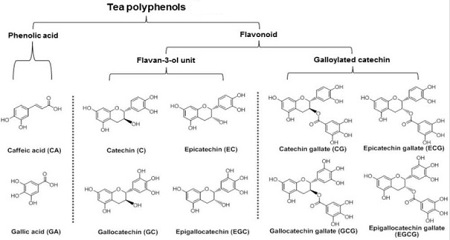 Figure 2 is the tea polyphenols containing the major chemical substances |
| Biological activity | Oral biological activity is poor; the efficacy of taking a dosage of 800 mg is actually an order of magnitude lower than the real situation. |
| Toxicity | EGCG might be carcinogenic; study has found that during pregnancy, intake of polyphenols will increase the risk of neonatal leukemia; it is not good for pregnant women to uptake bioflavones; during pregnancy, administration of tea or coffee during pregnancy may increase the risk for children of suffering from malignant tumor of central nervous system (CNS), and the specific mechanism remains unknown. |
| Biosynthesis pathway of Epicatechin gallate | 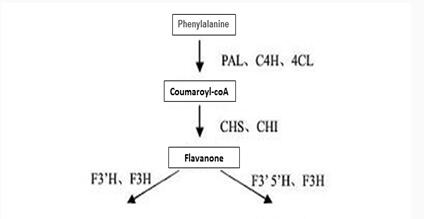 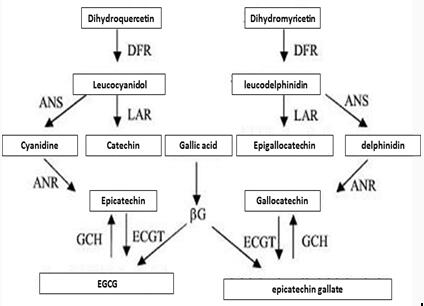 Figure 3 shows the synthesis of Epicatechin gallate in plants. Enzymes involved in the synthesis pathway include phenylalanine ammonialyase (PAL), cinnamate 4-hydroxylase (C4H), 4-¬coumaroyl¬CoA ligase (4CL), chalcone isomerase (CHI), chalcone synthase (CHS), flavonoid3’¬hydroxylase (F3’H),flavonoid3’, 5’¬hydroxylase (F3’5’H), (2S)¬flavanone 3¬hydroxylase (F3H), dihydroflavonol-4-¬reductase (DFR), leucoanthocyanidin reductase (LAR), anthocyanidin reductase,ANR、anthocyanidin synthase (ANS) and UDPG¬ flavonoid glucosyl transferase UFGT and so on. |
| Antibacterial effect | Epicatechin gallate has antibacterial effect, having varied bacteriostatic and bactericidal effect against many kinds of common pathogens including Proteus, Staphylococcus aureus, Staphylococcus epidermidis; Streptococcus, Botox, Lactobacillus and Vibrio cholera, especially intestinal pathogens. Moreover, epicatechin gallate can also effectively prevent the infection of antibiotic-resistant staphylococcus; it has inhibitory effect against hemolysin ECG and EGCG. In addition, it also has inhibitory effect against pathogenic fungi that can cause human skin disease, such as the head tinea alba, plaque blister white ringworm, sweat bubble white ringworm and stubborn tinea and other parasitic fungi. |
| Anti-oxidization effect | Epicatechin gallate belongs to a polyphenol hydroxyl chemical, and is quite easily to be oxidized into esters and provide proton H, thus having significant antioxidant property. The antioxidant capacity of tea polyphenols is 18 times than that of vitamin E; 3 to 10 times than that of vitamin C. It has the ability to block the synthesis of N-nitroso compounds, inhibit the activity of lipoxygenase and lipid peroxidation. This makes it play excellent disease prevention and treatment effect in the anti-cancer, anti-mutation, anti-aging, prevention and treatment of cardiovascular disease, treatment of hepatitis and many other aspects. |
| Other pharmacological effects | Hypoglycemic effect, a number of experimental data have confirmed that tea polyphenols is an invertase inhibitor, so it can inhibit the conversion of sucrose to glucose, leading to the decrease of the blood sugar.Antiviral effect, it has inhibitory effect against influenza A, influenza B virus as well as the human respiratory system covariate virus (RSV). In addition, tea polyphenols also have a strong anti-inhibitoryeffect against gastroenteritis virus, hepatitis A virus and plant viruses. In recent years, it has been confirmed that tea polyphenol is a strong novel inhibitor of HIV-IRT.Anti-cancer, anti-mutation effect; epicatechin gallate not only inhibits a variety of chemical carcinogen-induced mutations, but also inhibit the mutagenic effect of some kinds of mixed carcinogens (tobacco fog thickener, coal tar, smoked fish extract, X Ray).For the treatment of cardiovascular disease; studies have shown that tea polyphenols have various effects including anticoagulant, promote fibrinolysis, anti-platelet aggregation, lowering blood pressure, lowering blood pressure, prevention and treatment of atherosclerosis as well as protecting the myocardium.Anti-allergic and anti-inflammatory effect, tea polyphenols has a significant inhibitory effect on the transparent enzyme, among which theaflavin gallate has an inhibitory activity of 99.1%, being able to effectively inhibit the rapid allergic reaction.Gastrointestinal protection function. It can inhibit the H-K-ATPase in the gastric mucosa, which fundamentally inhibits the secretion of gastric acid. This reduces the gastric irritation and injury by gastric acid. It can treat ulcers and alleviate the gastrointestinal spasm as well. |
| Storage | It should be sealed, placed in a cool dry environment, to be avoided of moisture, light and high temperature. |
| Chemical Properties | White powder |
| Uses | An antioxidant investigated as an adjunct treatment of some methyicillin resistant bacteria. |
| Uses | (-)-Epicatechin gallate is polyphenols which are abundant in green and black teas. (-)-Epicatechin gallate (ECG) is a natural catechin with a single galloyl group. The hydroxyl groups of ECG contribute to its potent antioxidant activity and facilitate the killing of methicillin-resistant strains of S. aureus. |
| Uses | One of the catechin isomers and a potent antioxidant that can modulate a wide range of membrane proteins. Its bilayer-modifying potency was tested using gramicidin A (gA) channels as probes. All the c atechins alter gA channel function and modify bilayer properties, with a 500-fold range in potency. The gallate group causes current block, as evident by brief downward current transitions. |
| Definition | ChEBI: A gallate ester obtained by formal condensation of the carboxy group of gallic acid with the (3R)-hydroxy group of epicatechin. A natural product found in Parapiptadenia rigida. |
| General Description | (?)-Epicatechin gallate is one of the major polyphenols present in green tea. It belongs to the catechin gallates group of organic compounds. ECG has a chemical structure like that of (?)-epigallocatechin gallate (EGCG), the other major polyphenol found in green tea. |
| Biological Activity | (-)-epicatechin gallate is a major catechin component in green tea [1].(-)-epicatechin gallate (ecg) plays an important role in cell growth inhibition, apoptosis and membrane transport system [1].(-)-epicatechin gallate is a kind of catechin. in hct-116 cells, ecg activated transcription factor 3 (atf3), which played a critical role in pro-apoptosis. egr-1 was involved in ecg-induced atf3 expression. in hct-116 cells, ecg (50 μm) increased nag-1 and atf3 expression in time- and dose-dependent way [1]. in carcinoma hsc-2 cells, ecg (50 μm) exhibited cytotoxicity with midpoint cytotoxicity (nr50) value of 67 μm. however, in normal hgf-2 fibroblasts, ecg exhibited cytotoxicity at concentrations up to 25 μm with nr50 value of 100 μm. in carcinoma hsc-2 cells, ecg (250 μm) induced nucleosomal dna fragmentation and apoptosis. ecg (150 μm) significantly increased caspase-3 activity [2].in rats, there were five metabolites of ecg: (-)-epicatechin gallate, 3’,4’’-di-o-methyl-(-)-epicatechin gallate, 4’’-o-methyl-(-)-epicatechin gallate, 4’-o-methyl-(-)-epicatechin gallate and 3’-o-methyl-(-)-epicatechin gallate, which were excreted in rat urine [3]. |
| Biochem/physiol Actions | (?)-Epicatechin gallate (ECG), a potent free-radical scavenger has an inhibitory effect on collagenase and elastase enzymes making it an anti-aging agent. It displays antiproliferative effects on human colorectal cells and hence is a chemo-preventative agent. It influences cell survival, preventing apoptosis or autophagy by promoting cell proliferation in oxygen-glucose deprived human brain microvascular endothelial cells. Being structurally and functionally similar to EGCG, ECG might be involved in balancing the mammalian target of rapamycin (mTOR)-AMP-activated protein kinase (AMPK) pathways in endoplasmic reticulum stress. It prevents cell death in this scenario by showing resistance against oxygen-deprived cells. ECG may contribute in preventing oxidative stress. It effectively inhibits secretory sphingomyelinase in many disease states and provides protection for human spermatozoa in Assisted reproductive technology (ART). |
| references | [1]. cho kn, sukhthankar m, lee sh, et al. green tea catechin (-)-epicatechin gallate induces tumour suppressor protein atf3 via egr-1 activation. eur j cancer, 2007, 43(16): 2404-2412. [2]. babich h, krupka me, nissim ha, et al. differential in vitro cytotoxicity of (-)-epicatechin gallate (ecg) to cancer and normal cells from the human oral cavity. toxicol in vitro, 2005, 19(2): 231-242. [3]. kohri t, suzuki m, nanjo f. identification of metabolites of (-)-epicatechin gallate and their metabolic fate in the rat. j agric food chem, 2003, 51(18): 5561-5566. |
| (-)-Epicatechin gallate Preparation Products And Raw materials |