| Chemical Properties | orange crystals or powder |
| Uses | Collagen stain for connective tissue. Used with Orcein, Alizarin Blue 2B, and Fast Green FCF in Kornhauser′s quadruple stain for most elementary structures of tissues. |
| Uses | Orange G is an azo dye used primarily as a histological stain. Dyes and metabolites. |
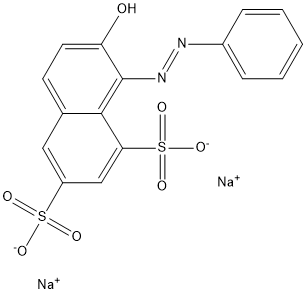
| Preparation | aniline diazo, and 7-Hydroxynaphthalene-1,3-disulfonic acid coupling. |
| Definition | ChEBI: An organic sodium salt that is the disodium salt of 7-hydroxy-8-[(E)-phenyldiazenyl]naphthalene-1,3-disulfonic acid. It is often combined with other yellow dyes in alcoholic solution to stain erythrocytes in trichrome methods, and is used or demonstrating cells in the pancreas and pituitary. |
| General Description | Orange microcrystals or powder. |
| Air & Water Reactions | Azo dyes can be explosive when suspended in air at specific concentrations. This organic acid has a moderate soluble in water. The resulting solutions contain concentrations of hydrogen ions and have pH’s of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. |
| Reactivity Profile | Acid Orange 10 is an azo compound. Toxic gases are formed by mixing compounds containing azo groups with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. Acid Orange 10 becomes redder and more dull when mixed with copper. Acid Orange 10 is almost destroyed when mixed with iron. |
| Health Hazard | ACUTE/CHRONIC HAZARDS: When heated to decomposition Acid Orange 10 emits toxic fumes of carbon oxides, nitrogen oxides, sulfur oxides and disodium oxide. |
| Fire Hazard | Flash point data for Acid Orange 10 are not available; however, Acid Orange 10 is probably combustible. |
| Biochem/physiol Actions | Orange G is used in collagen stain for connective tissue. It is used with Orcein, Alizarin Blue 2B, and Fast Green FCF in Kornhauser′s quadruple stain for most elementary structures of tissues. It is also used along with acid fuchsin and light green in masson trichrome stain for the staining of cellular structures, red blood cells and muscle cells. |
| Purification Methods | Recrystallise this dye from 75% EtOH, dry it for 3hours at 110o and keep it in a vacuum desiccator over H2SO4. The free acid crystallises from EtOH or conc HCl in deep red needles with a green reflex. [Conant & Pratt J Am Chem Soc 48 2483 1923, Drew & Landquist J Chem Soc 292 1938, Beilstein 16 H 301, 16 I 305, 16 II 141, 16 III 327.] |
| Properties and Applications | bright orange. Soluble in water for orange, slightly soluble in ethanol (golden orange) and soluble fiber element, insoluble in other organic solvents. The strong sulfuric acid for yellow orange, diluted for yellow; In nitric acid solution for wine red, to orange. Its water solution and strong hydrochloric acid for yellow orange; Add nitric acid for wine red, to orange; Add thick sodium hydroxide solution for orange brown. Used for silk and wool products dyeing, also can dye paper and manufacturing ink, used in wood and color pencil manufacturing and biological dyeing.
StandardLight FastnessSoapingPersperation FastnessOxygen bleachingFastness to seawaterFadingStainFadingStainFadingStainISO4-51521521AATCC41121111 |