| 6-Bromoquinoline Basic information |
| Bromoquinoline Application and synthetic method |
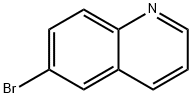
| Product Name: | 6-Bromoquinoline |
| Synonyms: | 6-Bromo-1-azanaphthalene;6-Bromoquinoline,97%;6-Br-quinoline;TIMTEC-BB SBB001559;6-bromo-quinolin;Quinoline, 6-bromo-;6-BROMOOQUINOLINE;6-BROMOQUINOLINE |
| CAS: | 5332-25-2 |
| MF: | C9H6BrN |
| MW: | 208.05 |
| EINECS: | 226-238-7 |
| Product Categories: | Aromatics;Heterocycles;Quinolines, Isoquinolines & Quinoxalines;Boronic Acid;Heterocyclic Compounds;Building Blocks;Miscellaneous;Quinoline Derivertives;Aromatics Compounds;Haloquinolines;quinoline;Halogenated Heterocycles;Heterocyclic Building Blocks;QuinolinesHeterocyclic Building Blocks;blocks;Bromides;Quinolines;Halides;Quinolines, Isoquinolines & Quinoxalines;Quinoline&Isoquinoline;5332-25-2 |
| Mol File: | 5332-25-2.mol |
 |
| 6-Bromoquinoline Chemical Properties |
| Melting point | 19°C |
| Boiling point | 116 °C / 6mmHg |
| density | 1.538 g/mL at 25 °C |
| refractive index | n20/D 1.663 |
| Fp | 19 °C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Soluble in acetone, acetonitrile, dichloromethane, ethyl acetate and THF. |
| pka | 4.18±0.10(Predicted) |
| form | Oil |
| color | Thick |
| InChIKey | IFIHYLCUKYCKRH-UHFFFAOYSA-N |
| CAS DataBase Reference | 5332-25-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 6-Bromo quinoline(5332-25-2) |
| EPA Substance Registry System | Quinoline, 6-bromo- (5332-25-2) |
| Safety Information |
| Hazard Codes | Xi,Xn |
| Risk Statements | 36/37/38-41-37/38-22-20/21/22 |
| Safety Statements | 26-37/39-39-36 |
| WGK Germany | 3 |
| TSCA | Yes |
| HazardClass | IRRITANT |
| HS Code | 29334900 |
| 6-Bromoquinoline Usage And Synthesis |
| Bromoquinoline | There are seven positional isomers of bromoquinoline and their main properties are listed below: 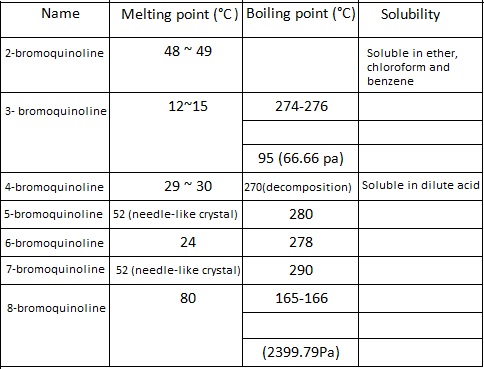 |
| Application and synthetic method | 3-bromo-quinoline is reacted with mixed acid for generating 3-bromo-5-nitro-quinoline, which heats together with potassium permanganate for being oxidation into 5-bromo-2, 3-pyridine dicarboxylic acid. 6-bromo-quinoline is heated together with nitric acid to generate 6-bromo-8-nitro quinolone with further reaction with potassium permanganate for being oxidized to 2, 3-pyridinedicarboxylic acid. 2-bromo-quinolien can be synthesized through the reaction between 2-hydroxy quinoline and phosphorus pentabromide. Quinoline perbromide is heated at 180 °C for generating 3-bromo-quinoline. From the heating between 4-hydroxy quinoline and phosphorus pentabromide, or from the diazotization reaction via 4-aminoquinoline to generate 4-bromo-quinoline; 5-bromo-quinoline is synthesize by the heating reaction between m-bromo-aniline, glycerol, m-bromo nitrobenzene and concentrated sulfuric acid, or from the diazotization reaction of 5-aminoquinoline. 6-bromo-quinoline can be synthesized from the heating of bromoaniline, glycerol, concentrated sulfuric acid, and p-bromo-nitrobenzene. 7-bromo-quinoline can be synthesized from the diazotization reaction of 7-aminoquinoline. 8-bromo-quinoline can be synthesized from the heating reaction of o-bromo-aniline, glycerol, concentrated sulfuric acid and o-bromo nitrobenzene. application: as organic synthesis reagents. The above information is edited by the Chemicalbook of Dai Xiongfeng. |
| Chemical Properties | Thick Oil |
| Uses | 6-Bromoquinoline is used as fine chemical and pharmaceutical intermediate, used as the coupling reagent. |
| 6-Bromoquinoline Preparation Products And Raw materials |