| Description | Trifloxystrobin is a synthetic derivative of the naturally occurring strobilurins found in several genera of wood-decaying fungi such as Strobilurus tenacellus. It is a strobilurin foliar fungicide. Trifloxystrobin inhibits mitochondrial respiration by blocking electron transfer within the respirator chain. Therefore, trifloxystrobin is a potent inhibitor of 2 fungal spore germination and mycelial growth. It has a high level of activity against many fungal pathogens within the Ascomycete, Deuteromycete, Basidiomycete, and Oomycete classes.
Pests controlled by trifloxystrobin include grape and cucurbit powdery mildew, apple scab and powdery mildew, peanut leafspot, and brown patch of turfgrass. It could be applied to cereals, ornamental, vegetables (carrots, asparagus, cucurbits, fruiting vegetables, root vegetables (except radish)), fruits (apples, pears, grapes, strawberries) and tropical crops. |
| References | [1] http://sitem.herts.ac.uk/aeru/iupac/Reports/664.htm
[2] EPA Pesticide Fact Sheet |
| Chemical Properties | Off-White to Pale Beige Solid |
| Uses | Trifloxystrobin is a broad-spectrum foliar fungicide used in plant pritection. Trifloxystrobin functions by inhibiting fungal spore germination. |
| Uses | Agricultural fungicide. |
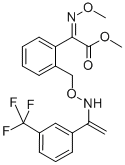
| Definition | ChEBI: Trifloxystrobin is the methyl ester of (2E)-(methoxyimino)[2-({[(E)-{1-[3-(trifluoromethyl)phenyl]ethylidene}amino]oxy}methyl)phenyl]acetic acid. A foliar applied fungicide for cereals which is particularly active against Ascomycetes, Deuteromycetes and Oomycetes It has a role as a mitochondrial cytochrome-bc1 complex inhibitor and an antifungal agrochemical. It is an oxime O-ether, an organofluorine compound, a methyl ester and a methoxyiminoacetate strobilurin antifungal agent. |
| Metabolism | Trifloxystrobin is described as “mesostemic” due to its ability to redistribute to untreated plant parts through vapor action, limited but effective cuticular penetration, and translaminarmovement by diffusion (30). It is rainfast by virtue of its high affinity for the waxy cuticular layer. |
| Toxicity evaluation | Trifloxystrobin has an acute oral LD50 > 5,000 and an acute dermal LD50 > 2,000 mg/kg in rats. It is not a skin or eye irritant, is nonmutagenic and nonteratogenic. It shows rapid absorption and elimination in the rat. It has no toxicity to birds in acute studies (LD50 > 2,000 mg/kg) but has an LC50 of 0.015 mg/L in rainbow trout. The bee LD50 = 200 μg/bee. Environmental fate studies show it to be hydrolytically stable at pH 5, with a DT50 of 11.4 weeks at pH 7. It has a photolytic DT50 of 31.5 h at pH 7 (25 ?C) in water, a soil adsorption coefficient (Koc) of 1,642-3,745 ml/g, and a soil DT50 of 5.4 days under field conditions. |