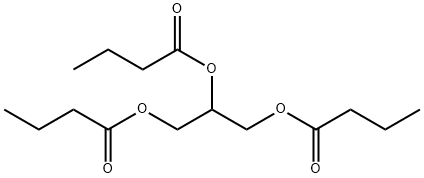
| Description | Tributyrin (C15H26O6), also known as butyrin or glyceryl tributyrate, is the triester of glycerin and butyric acid. It is prepared by esterifcation of glycerin with excess butyric acid. Glycerol tributyrate has a characteristic odor and a bitter taste.
Tributyrin is essentially a triacylglyceride (TAG), which is an ester derived from glycerol and 3 fatty acids. Tributyrin requires lipase to release the butyrate attached to the glycerol. Although 1 tributyrin contains 3 butyrate, not all 3 butyrate is guaranteed to be released. This is because lipase is regioselective. It can hydrolyse triacylglycerides at R1 and R3, only R2, or non-specifically. Lipase also has substrate specificity in that the enzyme can differentiate between acyl chains attached to the glycerol and preferentially cleaving certain types. Since tributyrin requires lipase to release its butyrate, there may be competition between tributyrin and other TAGs for lipase. |
| Chemical Properties | Tributyrin is a colorless, oily liquid with a bitter taste. It is soluble in alcohol and ether and fairly insoluble in water. |
| Chemical Properties | Glycerol tributyrate has a characteristic odor and bitter taste. |
| Uses | Tributyrin is a stable and rapidly absorbed prodrug of butyric acid which enhances antiproliferative effects of dihydroxycholecalciferol in human colon cancer cells. |
| Uses | Tributyrin is a flavoring agent that is the triester of glycerin and butyric acid. it is prepared by esterification of glycerin with excess butyric acid. it is used in the following foods: baked goods; alcoholic beverages; nonalcoholic beverages; fats and oils; frozen dairy des- serts and mixes; gelatins, puddings and fillings; and soft candy. it is also termed butyrin and glyceryl tributyrate. |
| Production Methods | Tributyrin is manufactured via esterification of glycerol with butyric acid. |
| Definition | ChEBI: A triglyceride obtained by formal acylation of the three hydroxy groups of glycerol by butyric acid. |
| Preparation | Prepared by esterifcation of glycerol with excess butyric acid. |
| Taste threshold values | Taste characteristics at 30 ppm: bitter, waxy, fatty, cheese and butter nuances. |
| General Description | Tributyrin is a short-chain triacylglycerol that mainly occurs in butter. It shows potent anti-cancer property. |
| Biochem/physiol Actions | Taste at 30 ppm |
| Safety Profile | Poison by intravenous route. Low toxicity by ingestion. Questionable carcinogen with experimental tumorigenic data. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS |
| Carcinogenicity | Administration of sodium butyrate in drinking water potentiates 1,2-dimethylhydrazine- induced colon cancer in rats . In mice, dietary administration of 5% tributyrin for 48 weeks did not lead to an increase in colonic tumor incidence or focal areas of dysplasia as compared to controls . Therefore, it has been suggested that the agent responsible for enhanced tumorigenesis in the rat study was sodium, rather than butyrate. |