| Tetramethylammonium fluoride Basic information |
| Product Name: | Tetramethylammonium fluoride |
| Synonyms: | Tetramethylammonium Fluoridede;n,n,n-trimethyl-methanaminiufluoride;TETRAMETHYLAMMONIUM FLUORIDE;Tetramethylammoniumfluoride,20%AqueousSolution;Tetramethylammonium fluoride, anhydrous;Methanaminium, N,N,N-trimethyl-, fluoride;Tetramethylazanium fluoride;Tetramethylaminium·fluoride |
| CAS: | 373-68-2 |
| MF: | C4H12FN |
| MW: | 93.14 |
| EINECS: | 206-769-0 |
| Product Categories: | Deprotecting Reagents;Fluoride Sources;Protection and Derivatization;quarternary ammonium salts |
| Mol File: | 373-68-2.mol |
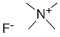 |
| Tetramethylammonium fluoride Chemical Properties |
| Melting point | 170 °C (dec.) (lit.) |
| InChIKey | HQFTZNVQVRRDLN-UHFFFAOYSA-M |
| CAS DataBase Reference | 373-68-2(CAS DataBase Reference) |
| EPA Substance Registry System | Tetramethylammonium fluoride (373-68-2) |
| Safety Information |
| Hazard Codes | Xn |
| Risk Statements | 20/21/22-36/37/38 |
| Safety Statements | 26-36 |
| RIDADR | 2811 |
| WGK Germany | 3 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29239000 |
| Provider | Language |
|---|---|
| Tetramethylammonium fluoride | English |
| Tetramethylammonium fluoride Usage And Synthesis |
| Description | Tetramethylammonium fluoride is the quaternary ammonium salt with the formula (CH3)4NF. This hygroscopic white solid is a source of “naked fluoride”, that is fluoride ions not connected to a metal atom in a complex. Most other soluble salts of fluoride are in fact bifluorides, HF2–. Historically, there has been two main approaches to prepare TMAF: (i) Via neutralization of tetramethylammonium hydroxide (TMAOH) with HF. (ii) through the metathesis reaction of different ammonium salts with inorganic sources of fluoride such as KF or CsF. Due to the high basicity of the fluoride anion, the salt reacts slowly with acetonitrile, inducing its dimerization to CH3C(NH2)=CHCN, which co-crystallizes. |
| Uses | Tetramethylammonium fluoride may be used in combination with sulfuryl fluoride for the conversion of aryl phenols to aryl fluorides. It can react with aminosilanes to generate onium amide bases in situ, which can deprotonate heteroarenes. |
| Uses | Reactant for: Halide-induced ring opening of 2-substituted aziridinium saltsSynthesis of fluoro aromatics via fluorodenitration reactionsSynthesis of sevoflurane in ionic liquids by halogen-exchange fluorinationSynthesis of fluorinated Poly(oxomolybdates) |
| Tetramethylammonium fluoride Preparation Products And Raw materials |