| Tetraethylammonium bromide Basic information |
| Product Name: | Tetraethylammonium bromide |
| Synonyms: | TETRAETHYLAMMONIUM BROMIDE;TEA BROMIDE;Ammonium, tetraethyl-, bromide;Beparon;Bromure de tetraethylammonium;tmd10;USAF do-32;usafdo-32 |
| CAS: | 71-91-0 |
| MF: | C8H20BrN |
| MW: | 210.16 |
| EINECS: | 200-769-4 |
| Product Categories: | Ammonium Salts;Greener Alternatives: Catalysis;Phase Transfer Catalysts;Ion-pair Reagents;Ammonium Bromides (Quaternary);Quaternary Ammonium Compounds;quarternary ammonium salts;Pharmaceutical intermediates;surfactant;bc0001 |
| Mol File: | 71-91-0.mol |
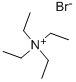 |
| Tetraethylammonium bromide Chemical Properties |
| Melting point | 285 °C (dec.)(lit.) |
| density | 1,397 g/cm3 |
| vapor density | 5.3 (vs air) |
| refractive index | 1,442-1,444 |
| storage temp. | Store below +30°C. |
| solubility | acetonitrile: 0.1 g/mL warm, clear, colorless |
| form | Liquid |
| color | White to cream |
| Odor | Odorless |
| PH Range | 6.5 at 100 g/l |
| PH | 6.5 (100g/l, H2O, 20℃) |
| Water Solubility | 2795 g/L (25 º C) |
| Sensitive | Hygroscopic |
| Merck | 14,9199 |
| BRN | 3563430 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | HWCKGOZZJDHMNC-UHFFFAOYSA-M |
| CAS DataBase Reference | 71-91-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Tetraethylammonium bromide(71-91-0) |
| EPA Substance Registry System | Ethanaminium, N,N,N-triethyl-, bromide (71-91-0) |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36-37/39 |
| WGK Germany | 3 |
| RTECS | BS5950000 |
| F | 3 |
| TSCA | Yes |
| HS Code | 29239000 |
| Provider | Language |
|---|---|
| Tetraethylammonium bromide | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Tetraethylammonium bromide Usage And Synthesis |
| Chemical Properties | white to light yellow crystalline solid |
| Uses | Tetraethylammonium bromide (TEAB) catalyzes the synthesis of thioesters by the oxidative coupling of aldehydes or alcohols with thiols or disulfides.It is used as a catalyst, along with o-iodoxybenzoic acid (IBX), in the oxidation of sulfides to sulfoxides and primary carboxamides to one-carbon dehomologated nitriles.TEAB can also be used as an organic template to synthesize zeolite beta. |
| Uses | Tetraethylammonium Bromide, can be used as a source of tetraethylammonium ions for various pharmaceutical studies. It has also the ability to block K+ channels in various tissues. |
| General Description | The use of ion-pairing reagents as mobile phase additives allows the separation of ionic and highly polar substances on reversed phase HPLC columns. The purity of mobile phase additives is of utmost importance to their successful application. Sigma-Aldrich offers an outstanding range of tailor-made reagents for anionic (quaternary ammonium and phosphonium salts) and cationic (alkanesulfonates) determination. All mobile phase additives are subject to rigorous testing with special emphasis on the requirements of modern reversed phase HPLC: |
| Purification Methods | Recrystallise the bromide from EtOH, CHCl3 or diethyl ether, or recrystallise it from acetonitrile and dry it over P2O5 under reduced pressure for several days. It also recrystallises from EtOH/diethyl ether (1:2), EtOAc, water or boiling MeOH/acetone (1:3) or by adding an equal volume of acetone and allowing to cool. Dry it at 100o in vacuo for 12 days, and store over P2O5. [Beilstein 4 IV 332.] |
| Tetraethylammonium bromide Preparation Products And Raw materials |
| Raw materials | Triethylamine–>Solvent |
| Preparation Products | Tetraethylammonium tetrafluoroborate–>Tetraethylammonium hexafluorophosphate–>Methyl bromide–>tetraethylammonium hydridotetracarbonylferrate |