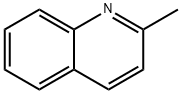
| Chemical Properties | Quinaldine or 2-methylquinoline is an organic compound with the formula CH3C9H6N. It is one of the methyl derivative of a heterocyclic compound quinoline. It is bioactive and is used in the preparation of various dyes. It is a colorless oil but commercial samples can appear colored. |
| Uses | It is an anaesthetic used in fish transportation. Used in preparation of oil-soluble dyes. Also used in anti-malaria drugs, in manufacturing dyes, food colorants , pharmaceuticals and pH indicators. |
| Uses | Quinaldine is an anaesthetic used in fish transportation. Also used in anti-malaria drugs, in manufacturing dyes, food colorants , pharmaceuticals and pH indicators. |
| Definition | ChEBI: A quinoline compound in which the quinoline skeleton is substituted at C-2 with a methyl group. |
| Synthesis Reference(s) | Journal of Heterocyclic Chemistry, 13, p. 1279, 1976 DOI: 10.1002/jhet.5570130626
The Journal of Organic Chemistry, 52, p. 3847, 1987 DOI: 10.1021/jo00226a023
Synthesis, p. 324, 1996 DOI: 10.1055/s-1996-4221 |
| General Description | A colorless oily liquid darkening to red-brown on exposure to air. Flash point 175°F. Denser than water and slightly soluble in water. Vapors heavier than air. Produces toxic oxides of nitrogen during combustion. Used to make dyes, pharmaceuticals and other chemicals. |
| Air & Water Reactions | Slightly soluble in water. |
| Reactivity Profile | Quinaldine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. Keep tightly closed and protected from light . |
| Hazard | Strong irritant to mucous membranes. |
| Fire Hazard | Quinaldine is probably combustible. |
| Purification Methods | Dry it with Na2SO4 or by refluxing with BaO, then fractionally distil it under reduced pressure and redistil it from zinc dust. Purify it further by conversion to its phosphate (m 220o) or picrate (m 192o) from which after recrystallisation, the free base is regenerated. [Packer et al. J Am Chem Soc 80 905 1958.] Its ZnCl2 complex can be used for the same purpose. [Beilstein 20 III/IV 3454, 20 V 375.] |