| Potassium oleate Basic information |
| Product Name: | Potassium oleate |
| Synonyms: | OLEIC ACID, POTASSIUM SALT, 40 WT. % PAS TE IN WATER;9-Octadecenoic acid (9Z)-, potassium salt;Kaliumoleat;OS Soap;PotassiuM oleate technical, >=87% (fatty acid);OLEIC ACID POTASSIUM SALT;POTASSIUM (Z)-9-OCTADECENOATE;POTASSIUM OLEATE |
| CAS: | 143-18-0 |
| MF: | C18H33KO2 |
| MW: | 320.55 |
| EINECS: | 205-590-5 |
| Product Categories: | Classes of Metal Compounds;K (Potassium) Compounds (excluding simple potassium salts);Typical Metal Compounds;Building Blocks;Carbonyl Compounds;Carboxylic Acid Salts;Chemical Synthesis;Organic Building Blocks |
| Mol File: | 143-18-0.mol |
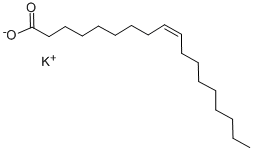 |
| Potassium oleate Chemical Properties |
| density | >1.1 g/cm3 |
| Fp | 140 °C |
| storage temp. | 2-8°C |
| Water Solubility | Soluble in water |
| form | powder to crystal |
| color | Pale white powder |
| Merck | 14,7650 |
| BRN | 4167152 |
| Hydrophilic-Lipophilic Balance (HLB) | 20 |
| InChIKey | MLICVSDCCDDWMD-KVVVOXFISA-M |
| LogP | 3.920 (est) |
| CAS DataBase Reference | 143-18-0(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium oleate (143-18-0) |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| RIDADR | UN 3077 9 / PGIII |
| WGK Germany | 1 |
| RTECS | RK1150000 |
| HS Code | 2916.15.5100 |
| Hazardous Substances Data | 143-18-0(Hazardous Substances Data) |
| Toxicity | eye-rbt 12 mg/48H JANCA2 56,905,73 |
| Provider | Language |
|---|---|
| Potassium oleate | English |
| SigmaAldrich | English |
| Potassium oleate Usage And Synthesis |
| Chemical Properties | Gray-tan paste. Soluble in water and alcohol. Combustible. |
| Uses | Potassium Oleate can be used as a compound disinfectant. |
| Uses | Potassium Oleate is the potassium salt of oleic acid. it is used as a binder, emulsifier, and anticaking agent. |
| General Description | Brown solid or clear to amber liquid with a soapy odor. Sinks and mixes slowly with water. |
| Air & Water Reactions | Water soluble. Gives basic aqueous solution. |
| Reactivity Profile | Salts, basic, such as OLEIC ACID, [POTASSIUM SALT], are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH’s greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. |
| Health Hazard | Inhalation of dust causes irritation of nose and throat, coughing, and sneezing. Ingestion causes mild irritation of mouth and stomach. Contact with eyes causes irritation. |
| Fire Hazard | Special Hazards of Combustion Products: Irritating vapors and toxic gases, such as carbon dioxide and carbon monoxide, may be formed when involved in fire. |
| Safety Profile | An eye irritant. When heated to decomposition it emits toxic fumes of K2O. |
| Purification Methods | Recrystallise it from EtOH (1g/mL). [Beilstein 2 H 465, 2 I 196, 2 I 202, 2 II 436, 2 III 1404, 2 IV 1646.] |
| Potassium oleate Preparation Products And Raw materials |
| Raw materials | Formaldehyde–>Dimethylamine |
| Preparation Products | SODIUM OLEATE–>Oleic acid |