| Uses | Penconazole is used for the control of powdery mildew, pome fruit scab and other pathogenic Ascomycetes, Basidiomycetes and Deuteromycetes on vines, cucurbits, pome fruit, stone fruit, ornamentals, hops, tobacco and vegetables. |
| Uses | Penconazole is an conazole based fungicide that works by inhibiting cell membrane ergosterol biosynthesis, thus stopping the development of fungi. |
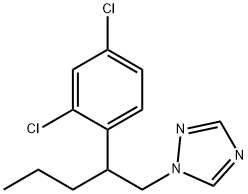
| Definition | ChEBI: 1-[2-(2,4-dichlorophenyl)pentyl]1,2,4-triazole is a member of the classof triazoles that is 1,2,4-triazole substituted at position 1 by a 2-(2,4-dichlorophenyl)pentyl group. It is a dichlorobenzene and a member of triazoles. |
| Agricultural Uses | Fungicide: Penconazole is a systemic fungicide used to control powdery mildew. It is used on apples and grapes and other fruits, hops, tobacco, ornamentals and on vegetables. Not currently registered in the U.S. Reported to be used in most European countries. Currently, there are more than 30 global suppliers. |
| Trade name | AWARD®; CGA-71818®; ONMEX®; TOPAS®; TOPAS-C®; TOPAS-MZ®; TOPAZ®; TOPAZE®; TOPAZE-C®; TOPENCO 100EC® |
| Safety Profile | Moderately toxic by ingestion and skin contact. When heated to decomposition it emits toxic vapors of NOx and Clí. |
| Metabolic pathway | Penconazole is metabolised in plants by hydroxylation of the propyl side chain and conjugation or metabolism to triazolylalanine and triazolylacetic acid. It is quite persistent in soils but degradation by sunlight requires only a few days. It is eliminated rapidly from mammals. |
| Degradation | Penconazole (1) is stable to hydrolysis at pH 1-13 and at temperatures up to 350 °C. It is degraded in sunlight with a DT50 of 4 days in natural sunlight. Its photochemical reactions have been studied. On irradiation with light of wavelengths greater than 280 nm (high-pressure mercury lamp with filter), the major reaction was the formation of a cyclised product (2) (Scheme 1). Irradiation under the same conditions in isopropanol gave mainly 2 and 3 (a dechlorinated product). After 8 hours, yields were 29.8% of 2 and 12.3% of 3 respectively. The product 3 alone photodegraded only slowly but 2 was completely photolysed within 3 hours to further products, indicating its role as an intermediate in the photolytic pathways (Schwack and Hartmann, 1992, 1994). In the presence of isopropanol, photodehalogenation of 3 to 4 competed with substitution of a solvent molecule to give a low yield ( |