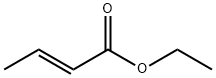
| Chemical properties | It appears as colorless to pale yellow liquid with the boiling point of 136 ° C and flash point of 22 ° C. It exhibits strong acid coke and fruit aroma with the incense of rum and ether.
The natural products exist in apples, papaya, strawberries, rums, wines and cocoa. |
| Application | It as allowable food spices. Mainly used for the preparation of fruit wine flavor.Used as the intermediate of organic synthesis, solvents, paint softener. |
| Production | It is obtained from the esterification of crotonic acid and ethanol in the presence of concentrated sulfuric acid. |
| Chemical Properties | colourless liquid |
| Chemical Properties | Ethyl crotonate has a powerful, sour, caramellic-fruity odor. |
| Occurrence | Reported found in Fragaria vesca. Also reported found in guava fruit, guava peel, pineapple, white wine, yellow passion fruit, fresh mango, naranjilla fruit, mussel, loganberry, apple, papaya, concord grape, strawberry, rum, cocoa, plum, kiwifruit and other natural sources. |
| Uses | Solvent and softening agent, lacquers, organic synthesis. |
| Uses | Ethyl crotonate is used as a solvent for cellulose esters as well as a plasticizer for acrylic resins. |
| Uses | Ethyl Crotonate is a synthetic flavoring agent that is a moderately stable, colorless to light yellow liquid of sharp winey note. it should be stored in glass, tin, or resin-lined containers. it is used in fruit flavors for application in baked goods, beverages, and candy at 2–7 ppm. |
| Preparation | By esterification of crotonic acid with ethyl alcohol in the presence of concentrated H2SO4. |
| Definition | ChEBI: Ethyl crotonate is a fatty acid ester. |
| Taste threshold values | Taste characteristics at 10 ppm: rum, cognac and pungent with caramellic and fruity nuances. |
| General Description | Ethyl trans-2-butenoate is a volatile flavor compound found in fruits such as cashew apple, African star apple, naranjilla fruit and sweet passion fruit. |
| Hazard | Flammable, dangerous fire risk. Strong irritant. |
| Safety Profile | Moderately toxic by ingestion and probably by inhalation. A skin , mucous membrane, and severe eye irritant. Very dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. To fight fire, use foam, CO2, or dry chemical. See also ESTERS. When heated to decomposition it emits acrid smoke and fumes. |
| Purification Methods | Wash it with aqueous 5% Na2CO3, then with saturated aqueous CaCl2, dry it with CaCl2 and distil it. [Beilstein 2 IV 1500.] |