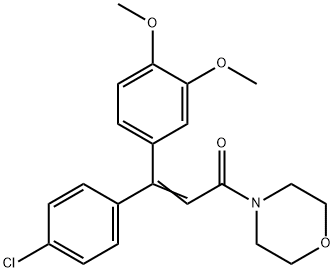
| Description | Dimethomorph is a cinnamic acid derivative and a member of the morpholine chemical family. Dimethomorph is a mixture of E- and Z-isomers in the ratio of about 1:1 and only the Z-isomer has the fungicidal activity. It fungicidal activity works by the inhibition of sterol (ergosterol) synthesis.
Dimethomorph is a systemic fungicide to protect against various fungal pathogens in vines and other crops. Dimethomorph is used to against downy mildews, late blights, anthracnose, septoria leaf spot, crown rot, and root rot for curbubits, grapevines, head lettuce, onions, potatoes, tomatoes, and fruit including blackberries, raspberries, strawberries. |
| References | [1] http://www.fao.org
[2] http://pmep.cce.cornell.edu
[3] http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/245.htm |
| Description | Dimethomorph is a morpholine fungicide that inhibits fungal cell wall formation. It inhibits mycelial growth of the oomycete fungi P. citrophthora, P. parasitica, P. capsici, and P. infestans (EC50s = 0.14, 0.38, <0.1, and 0.16-0.3 μg/ml, respectively) but is less active against the green algae species C. vulgaris or S. obliquus in vitro (EC50s = 47.46 and 44.87 μg/ml, respectively). It inhibits androgen receptor (AR) activity in a reporter assay in MDA-kb2 human breast cancer cells but not in a yeast antiandrogen screen (IC20s = 0.263 and 38.5 μM, respectively). It is not toxic to rats (LD50 = 3,900 mg/kg) or goldfish (C. auratus; LC50 = >32 μg/ml). |
| Chemical Properties | Off-White Solid |
| Uses | Agricultural fungicide. |
| Uses | Dimethomorph is a systemic fungicide which exhibits protectant, curative and antisporulant activities. It is active against fungal diseases such as leaf blight, downy mildew, damping off and foot-rot caused by Phytophthora spp. in/on grapes, potatoes and tomatoes. |
| Definition | ChEBI: A mixture of (E)- and (Z)-dimethomorph in an unspecified ratio. It is used as a systemic fungicide used on vines, potatoes, and greenhouse crops; only the Z isomer has fungicidal activity. |
| Agricultural Uses | Fungicide: A systemic fungicide that protects crops from mold. It also kills mold and prevents their spread; controls late blight on tomatoes; and is used as a wood preservative to control downey mildew. |
| Trade name | ACROBAT® WP; FORUM DC®, [manco- zeb + dimethomorph]; CME 151®; STATURE® |
| Metabolic pathway | Limited data are available in the open literature. Information presented in this summary is abstracted from the data evaluation published by the Pesticide Safety Directorate (PSD, 1994). Dimethomorph is a (1:l) mixture of the E- and Z-isomers. The Z-isomer has been shown to have all the intrinsic biological activities. The isomerisation of the E- and Z-isomers was observed when resolved isomers were exposed to UV light. Dimethomorph undergoes extensive degradation and metabolism in soil, plants and animals. The major metabolic pathway is the O-demethylation of one of the phenolic methoxy moieties to the corresponding phenols and conjugates. Other metabolic reactions include the oxidation of one of the CH2 moieties of the morpholine ring and the cleavage/opening of the morpholine ring (Scheme 1). |
| Degradation | Dimethomorph (1) is hydrolytically stable with no observable degradation in pH 4-9 buffered solutions at 70-90 °C for up to 10 weeks.
Degradation occurred when dimethomorph in pH 5 buffer solution was irradiated under Hanau Suntest apparatus (<290 nm) at 25 °C. The calculated aqueous photolytic degradation half-life (DT50) was ca. 25-28 days of continuous irradiation, No sigruficant degradation product (>7% of the applied radioactivity) was identified. |