| Description | Difenoconazole is a kind of triazole-type fungicide. It is a broad-spectrum triazole fungicide. It takes effect through acting as the inhibitor of sterol 14α-demethylase, blocking the biosynthesis of sterol. Through inhibiting the sterol biosynthesis process, it inhibits the mycelia growth and germination of pathogens by spores, ultimately suppressing the proliferation of fungi. Difenoconazole has been extensively used in a wide range of crops in many countries due to its ability to control various fungal diseases. It is also one of the most important and widely-used pesticides for disease control in rice. |
| Uses | Agricultural fungicide. |
| Uses | Pestanal is an fungicide that exhibits a broad spectrum of activities against a wide variety of fungi including members of the Aschomycetes, Basidomycetes and Deuteromycetes families. |
| Uses | Difenoconazole is a fungicide with broad-range activity, protecting yield and quality by foliar application or seed treatment. It provides long-lasting and curative activity against Ascomycetes, Basidiomycetes and Deuteromycetes. It is used against disease complexes in grapes, pome fruit, stone fruit, potatoes, sugar beet, oilseed rape, banana, ornamentals and various vegetable crops. It is also used as a seed treatment against a range of pathogens in wheat and barley. |
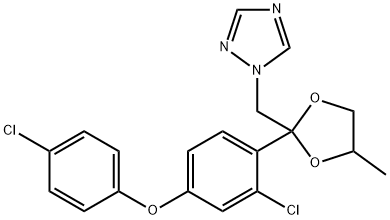
| Definition | ChEBI: A member of the class of dioxolanes that is 1,3-dioxolane substituted at position 2 by 2-chloro-4-(4-chlorophenoxy)phenyl and 1,2,4-triazol-1-ylmethyl groups. A broad spectrum fungicide with novel broad-range activity used as a spray or seed treatment. It is moderately toxic to humans, mammals, birds and most aquatic organisms. |
| Agricultural Uses | Fungicide: For suppression of fungi diseases in crops and seeds. |
| Trade name | CGA 169374®; DIVIDEND®; DIVIDEND® EXTREME FUNGICIDE; HELIX®; SCORE®; TECHNICAL CGA-169374® |
| Metabolic pathway | There is limited published information on the metabolism of difenoconazole. It is slowly dissipated in soils, and metabolism in plants involves rupture of the triazole linkage or oxidation of the phenyl ring followed by conjugation. |
| Degradation | Difenoconazole is stable to hydrolysis and is thermally stable to 150 °C. DT50 of difenocoazole in natural sunlight was 145 days. |
| References | Kwok, Iris M‐Y., and R. Thomas Loeffler. “The biochemical mode of action of some newer azole fungicides.” Pest Management Science 39.1 (1993): 1-11.
Wang, K., J. X. Wu, and H. Y. Zhang. “Dissipation of difenoconazole in rice, paddy soil, and paddy water under field conditions.” Ecotoxicology and environmental safety 86 (2012): 111-115. |