| Description | Diafenthiuron is a broad spectrum acaricide and insecticide, diafenthiuron is registered for use on cotton, soybeans, vegetables, fruit and ornamentals. It controls all post-hatch stages of mites, whiteflies and aphids. Its ability to control all sucking pests of cotton as well as mites, with no known toxicity to beneficial insects, is unique and very valuable in cotton integrated pest management (IPM) programs. |
| Uses | Diafenthiuron is an insecticide. |
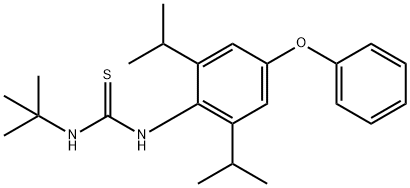
| Definition | ChEBI: Diafenthiuron is an aromatic ether that is 1,3-diisopropyl-5-phenoxybenzene in which the hydrogen atom at position 2 is substituted by a (tert-butylcarbamothioyl)nitrilo group. An agricultural proinsecticide which is used to control mites, aphids and whitefly in cotton. It has a role as an oxidative phosphorylation inhibitor and a proinsecticide. It is a thiourea acaricide, a thiourea insecticide and an aromatic ether. It is functionally related to a diphenyl ether. |
| Toxicology | Diafenthiuron has low toxicity to birds, mammals and beneficial insects, and is only slightly toxic to predatory mites. It is toxic to fish, but presents little hazard because it is rapidly degraded. |
| Mode of action | Diafenthiuron is a proinsecticide, which means that it must be converted to another compound – the drug – in order to be toxic. Activation of diafenthiuron to its carbodiimide drug occurs on the leaf surface, catalyzed by light, or in the insect, catalyzed by P450 monooxygenases. Diafenthiuron carbodiimide binds to the glutamate residue in the transmembrane F0 subunit of ATP synthase where protons from the intermembrane space dock to begin their journey across the inner mitochondrial membrane. Binding of diafenthiuron carbodiimide to this site blocks proton transport and ATP synthesis. Diafenthiuron was launched in 1991 and resistance has not yet been reported. |