| Benzyltriethylammonium chloride Basic information |
| Product Name: | Benzyltriethylammonium chloride |
| Synonyms: | BenzeneMethanaMiniuM,N,N,N-triethyl-, chloride (1:1);Benzenemethanaminium,N,N,N-triethyl-,chloride;benzyltriethyl-ammoniuchloride;Benzyltriethylammoniumchlor;n,n,n-triethyl-benzenemethanaminiuchloride;N,N,N-Triethylbenzenemethanammonium chloride;BenzylTriethylAmmoniumChlorideForSynthesis;BerylliumSulfate(Tetrahydrate)(Lab) |
| CAS: | 56-37-1 |
| MF: | C13H22ClN |
| MW: | 227.77 |
| EINECS: | 200-270-1 |
| Product Categories: | Quaternary ammonium salt;Pharmaceutical Intermediates;Ammonium Chlorides (Quaternary);Quaternary Ammonium Compounds;Ammonium Salts;Greener Alternatives: Catalysis;Phase Transfer Catalysts;Resin additives;56-37-1 |
| Mol File: | 56-37-1.mol |
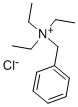 |
| Benzyltriethylammonium chloride Chemical Properties |
| Melting point | 190-192 °C (dec.) (lit.) |
| Boiling point | 366.11°C (rough estimate) |
| density | 1.08 g/mL at 25 °C |
| refractive index | n20/D 1.479 |
| Fp | >100°C |
| storage temp. | Store below +30°C. |
| solubility | H2O: 0.1 g/mL, clear |
| form | Liquid |
| color | White to beige |
| PH | 6-8 (100g/l, H2O, 20℃) |
| PH Range | 6 – 8 |
| Water Solubility | 700 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Decomposition | 185°C |
| BRN | 3574984 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| InChIKey | HTZCNXWZYVXIMZ-UHFFFAOYSA-M |
| CAS DataBase Reference | 56-37-1(CAS DataBase Reference) |
| EPA Substance Registry System | Benzyltriethylammonium chloride (56-37-1) |
| Safety Information |
| Hazard Codes | Xn,Xi |
| Risk Statements | 36/37/38-20/21/22 |
| Safety Statements | 26-36-37/39-26 37/39-36/37/39 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | BO8400000 |
| F | 3 |
| Autoignition Temperature | 300 °C |
| TSCA | Yes |
| HS Code | 29239000 |
| Toxicity | LD50 orally in Rabbit: 2219 mg/kg |
| Provider | Language |
|---|---|
| N,N,N’-Triethylbenzenemethanaminium chloride | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Benzyltriethylammonium chloride Usage And Synthesis |
| Chemical Properties | white to light yellow crystal powder. soluble in water, aqueous solutions conduct electricity. It is hydrochloride salt of benzyltriethylammonium which acts as a phase-transfer catalyst in chemical reactions. |
| Uses | Benzyltriethylammonium chloride is used as a catalyst in the preparation of 2-phenylbutyronitrile from phenyl acetonitrile. It is involved in the Knoevenagel condensation of carbonyl compounds with active methylene compounds to give olefinic products. It acts as a phase transfer catalyst used in the alkylation reaction. It reacts with 1H-Pyridine-2-thione to get 2-Benzylsulfanyl-pyridine. |
| Preparation | Benzyltriethylammonium chloride synthesis: Add benzyl chloride, triethylamine and acetone into the reaction pot, and reflux at 63-64°C for 8 hours. Slowly lowered to 15°C, filtered, and the filter cake was washed with acetone and dried to obtain benzyltriethylammonium chloride. Yield 68.9%. |
| Application | Benzyltriethylammonium chloride is a lipophilic phase-transfer catalyst that can be used in phase-transfer catalysis (PTC) to catalyze polycondensation reactions to form high molecular weight polymers under bi-phasic conditions. It can also be used: To activate hydroxyapatite and natural phosphate for use as a solid support for Knoevenagel condensation and Claisen-Schmidt condensation, respectively at room temperature and in the absence of a solvent. To increase the efficiency of mCPBA oxidation of sulfonimine generated from arenesulfonamide and diethyl acetal of an aromatic aldehyde to form 2-sulfonyloxaziridines. In combination with antimony(V) chloride to form a catalytic system for the Friedel-Crafts acylation reactions of arenes with acyl and sulfonyl chlorides. |
| Safety Profile | Poison by intravenous route.When heated to decomposition it emits toxic vapors ofNOx and Cl. |
| Benzyltriethylammonium chloride Preparation Products And Raw materials |