| Chemical Properties | Benzyldimethylstearylammonium Chloride is a light yellow sticky paste. It is soluble in water, acid resistant, hard water resistant, inorganic salt resistant, not alkali resistant. |
| Uses | Stearyldimethylbenzylammonium chloride is an anti-static that can also be used as a surfactant and/or a preservative depending on formulatory requirements. |
| Uses | Stearyldimethylbenzylammonium chloride is a compound used in products for promoting skin cleanliness and health. It is used in hair care formulations, as a hair conditioner and cream rinse. |
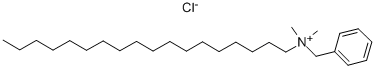
| Preparation | Synthesis of benzyldimethylstearylammonium chloride: put 450kg of dimethyl octadecylamine into the reaction kettle, heat to 80~85℃, and slowly add 180kg of benzyl chloride under stirring in 1.5~2h. Keep warm at 80~90℃ during the drop addition. After the dosing is completed, increase the temperature to 100~105℃, hold the reaction for several hours and take samples to measure the pH value. When the pH value of 1% aqueous solution reaches 6~6.5, the reaction is completed. Cool down to 60℃ and discharge to get benzyldimethylstearylammonium chloride. |
| General Description | Stearyldimethylbenzylammonium chloride is White solid or thick liquid with a mild odor. |
| Air & Water Reactions | Insoluble in water. |
| Reactivity Profile | Acidic organic salt solutions contain moderate concentrations of hydrogen ions and have pH’s of less than 7.0. They react as acids to neutralize bases. |
| Health Hazard | Ingestion causes gastrointestinal disturbances. Contact with chemical irritates eyes and skin and may damage eyes. |
| Fire Hazard | Special Hazards of Combustion Products: Toxic oxides of nitrogen and hydrochloric acid fumes may form in fires. |
| Flammability and Explosibility | Notclassified |
| Safety Profile | Poison by intraperitoneal route. Moderately toxic by ingestion. A human skin irritant and severe experimental eye irritant. When heated to decomposition it emits very toxic fumes of NOx, NH3, and Cl-. |
| Purification Methods | Crystallise the salt from acetone, EtOAc or EtOAc/ Et2O. [Sumiki et al. J Agric Chem Soc Jpn 26 325 1952, Chem Abstr 3505 1953, Beilstein 12 III 2212, 12 IV 2168.] |