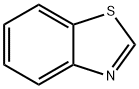
| Description | Benzothiazole and its derivative are recognized as the most important heterocyclic compounds. This series of compounds is of particular interests. It has various kinds of pharmacological properties and has been included in many kinds of natural products and pharmaceutical agents. The broad spectrum of pharmacological activity in individual benzothiazole derivative enables it to serve as unique and valuable scaffolds for experimental drug design. It is already known that benzothiazole and its derivative has various biological applications including anticancer, antimicrobial, anticonvulsant, antiviral, antidiabetic, antipsychotic, antiinflammatory, analgesic, fungicidal and diuretic. Its derivatives also have many applications in polymer chemistry, dyes, drugs, and silver photography as well as rubber industry. |
| Chemical Properties | yellow liquid with an unpleasant odour |
| Chemical Properties | Benzothiazole has a delicate, persistent, rose-like odor similar to that of quinoline. |
| Occurrence | Reported found in apricot, cooked asparagus, mozzarella cheese, skim milk powder, roasted beef, beer, malt whiskey, coconut meat, fresh mango, cooked broccoli and kelp. |
| Uses | It is an interesting carbonyl equivalent and reacts with aldehydes or ketones to generate α-hydroxy carbonyl compounds. Benzothiazole is used as a chemical intermediate in organic synthesis. It is a precursor of rubber accelerators and a component of cyanine dyes. It is also used as a flavoring substance. It is also used as a flavor, antimicrobial agent, and component of cyanine dyes. Its derivatives are used as rubber accelerators. |
| Uses | In organic synthesis. |
| Uses | Various benzothiazole-benzamides synthesized were evaluated for their analgesic and antidepressant activities. |
| Definition | ChEBI: An organic heterobicyclic compound that is a fusion product between benzene and thiazole. The parent of the class of benzothiazoles. |
| Preparation | By refluxing a mixture of zinc o-aminophenylsulfide and formic acid, followed by steam distillation of the alkalized reaction mixture; by heating formanilid or dimethylaniline with sulfur; by oxidation of 2-mercaptobenzothiazole or of the corresponding disulfide. |
| Aroma threshold values | Detection: 80 to 450 ppb |
| Taste threshold values | Taste characteristics at 3 ppm: meaty, vegetative, brown, cooked, beefy and coffee-like. |
| Synthesis Reference(s) | Journal of the American Chemical Society, 77, p. 6559, 1955 DOI: 10.1021/ja01629a041 |
| General Description | Benzothiazole, a volatile sulfur-containing heterocyclic compound, is one of the key odorants of heated milk. It is also reported to occur as a flavor component of miso, coconut meat and cooked-beef. |
| Hazard | Highly toxic by ingestion. |
| Safety Profile | Poison by ingestion,intraperitoneal, intravenous, and possibly other routes.When heated to decomposition it emits very toxic fumesof SOx, CN??, and NOx. |
| References | Gupta, Akhilesh, and S. Rawat. “Synthesis and cyclization of benzothiazole.” J. of Current Pharmaceutical Research 3.1 (2010): 13-25.
Keri, Rangappa S., et al. “A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry.” European journal of medicinal chemistry 89 (2015): 207-251.
Khokra, Sukhbir L., et al. “Common methods to synthesize benzothiazole derivatives and their medicinal significance: a review.” International Journal of Pharmaceutical Sciences and Research 2.6 (2011): 1356. |