| Benzalkonium chloride Basic information |
| Product Name: | Benzalkonium chloride |
| Synonyms: | BENZALKONIUM CHLORIDE, BP 99+%;BenzalkoniumChlorideB.P.;BenzalkoniumChloridePure;BENZALKONIUM CHLORIDE SOLUTION 10%;BENZALKONIUM CHLORIDE SOLUTION 50%, PHARMA;BENZALKONIUM CHLORIDE, POWDER;BENZALKONIUM CHLORIDE, GEL;Benzalkonium chloride (non-medicinal) |
| CAS: | 8001-54-5 |
| MF: | C17H30ClN |
| MW: | 283.88 |
| EINECS: | 616-786-9 |
| Product Categories: | Quaternary ammonium salt;CELEBREX;Benzaltex、Pharmatex;HYAMINE;BAC;Water treatment;cosmetic;Disinfectants;bc0001;8001-54-5 |
| Mol File: | 8001-54-5.mol |
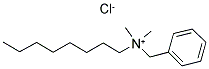 |
| Benzalkonium chloride Chemical Properties |
| Boiling point | >100°C/760mmHg |
| density | 0.98 |
| storage temp. | Store below +30°C. |
| solubility | Very soluble in water and in ethanol (96 per cent). An aqueous solution froths copiously when shaken. |
| form | Viscous Solution |
| Specific Gravity | 0.98 |
| color | Clear or white to yellow, may contain yellow-white fragments |
| Stability: | Stable. Incompatible with strong oxidizing agents, moisture. Hygroscopic. |
| LogP | 1.692 (est) |
| CAS DataBase Reference | 8001-54-5(CAS DataBase Reference) |
| EPA Substance Registry System | Benzalkonium chloride (8001-54-5) |
| Safety Information |
| Hazard Codes | C,N |
| Risk Statements | 21/22-34-50 |
| Safety Statements | 36/37/39-45-61 |
| RIDADR | UN 3261 8/PG 3 |
| WGK Germany | 3 |
| RTECS | BO3151000 |
| F | 3-9 |
| HS Code | 34021200 |
| Hazardous Substances Data | 8001-54-5(Hazardous Substances Data) |
| Toxicity | LD50 orally in rats: 400 mg/kg (Cummins, Kimura) |
| Provider | Language |
|---|---|
| Alkyldimethylbenzylammonium chloride | English |
| Benzalkonium chloride Usage And Synthesis |
| Description | Benzalkonium chloride is a quaternary ammonium mixture of alkyl, dimethyl and benzyl ammonium chlorides.It is an irritant rather than a sensitizer, but may cause contact dermatitis in nursing and medical personnel. An unusual case was reported in a farm worker who used a detergent containing benzalkonium chloride to clean the piggery. |
| Chemical Properties | white or light yellow/grey solid, or colourless aqueous solution |
| Chemical Properties | Benzalkonium chloride occurs as a white or yellowish-white amorphous powder, a thick gel, or gelatinous flakes. It is hygroscopic, soapy to the touch, and has a mild aromatic odor and very bitter taste. |
| Uses | benzalkonium chloride is a preservative with anti-microbial and deodorant properties. It can also be used as a surfactant. With continuous use, it may cause occasional allergic reactions. |
| Uses | Cationic surface active agent and germicide. Pharmaceutic aid (preservative). Algicide. |
| Uses | Benzalkonium chloride is a quaternary ammonium antiseptic and disinfectant with actions and uses similar to those of other cationic surfactants. It is used prior to surgical procedures or for minor wound care to reduce risks of infection. For most multidose aqueous nasal, ophthalmic and otic products, benzalkonium chloride is the preservative of choice. |
| Definition | ChEBI: A class of quaternary ammonium chloride salts in which the nitrogen is substituted by a benzyl group, two methyl groups and an even-numbered alkyl chain. |
| Production Methods | Benzalkonium chloride is formed by the reaction of a solution of Nalkyl- N-methylbenzamine with methyl chloride in an organic solvent suitable for precipitating the quaternary compound as it is formed. |
| Brand name | Roccal (Sterling Winthrop); Zephiran Chloride (Sterling Winthrop). |
| Hazard | Highly toxic; poison. |
| Agricultural Uses | Biocide, Algaecide, Fungicide: Used as an algaecide to control slime mold, algae, fish pathogens, and mollusks in ponds, canals and bodies of water. Also widely used in deodorants, detergents, sanitizers and germicides for applications in food plants, laundries, and operating rooms. Not listed for use in EU countries . Registered for use in the U.S. but not California. |
| Pharmaceutical Applications | Benzalkonium chloride is a quaternary ammonium compound used in pharmaceutical formulations as an antimicrobial preservative in applications similar to other cationic surfactants, such as cetrimide. In ophthalmic preparations, benzalkonium chloride is one of the most widely used preservatives,at a concentration of 0.01–0.02% w/v. Often it is used in combination with other preservatives or excipients, particularly 0.1% w/v disodium edetate, to enhance its antimicrobial activity against strains of Pseudomonas. In nasal, and otic formulations a concentration of 0.002–0.02% w/v is used, sometimes in combination with 0.002–0.005% w/v thimerosal. Benzalkonium chloride 0.01% w/v is also employed as a preservative in small-volume parenteral products. Benzalkonium chloride was also shown to enhance the topical penetration of lorazepam. Benzalkonium chloride is additionally used as a preservative in cosmetics. |
| Trade name | AMMONYX®; ARQUAD DMMCB-75®; BARQUAT® MB-50; BARQUAT® MB-80; BAYCLEAN®; BIO-QUAT 50-24®; CATAMINE® AB; CONSAN®; DRAPOLENE®; GARDIQUAT®-1450; HYAMINE®-3500; INTEXAN® LB-50; KATAMINE® AB; NEO GERM-I-TOL®; ONYX BTC; OSVAN; RODALON®; SENTINEL®; TRITON® K-60; VIKROL® RQ |
| Contact allergens | This quaternary ammonium cationic surfactant is a mixture of alkyl, dimethyl, and benzyl ammonium chlorides (-R). It is an irritant rather than a sensitizer, but may cause allergic contact dermatitis from creams, detergents/antiseptics, ophthalmic preparations, and in nursing, veterinary, dental, and medical personnel. Its presence was observed in plaster of Paris. |
| Clinical Use | Alkylbenzyldimethylammonium chloride (Zephiran) is a mixtureof alkylbenzyldimethylammonium chlorides of the generalformula [C6H5CH2N(CH3)2R] Cl , where R represents amixture of alkyl chains beginning with C8H17 and extendingto higher homologues with C12H25, C14H29, and C16H33. The higher–molecular-weight homologues compose the majorfractions. Although variations in the physical and antimicrobialproperties exist between individual members of the mixture,they are of little importance in the chemistry of the overallproduct. Benzalkonium chloride occurs as a white gel thatis soluble in water, alcohol, and organic solvents. Aqueous solutionsare colorless, slightly alkaline, and very foamy. Benzalkonium chloride is a detergent, an emulsifier, anda wetting agent. It is used as an antiseptic for skin and mucousmembranes in concentrations of 1:750 to 1:20,000. Forirrigation, 1:20,000 to 1:40,000 concentrations are used. Forstorage of surgical instruments, 1:750 to 1:5,000 concentrationsare used, with 0.5% NaNO3 added as a preservative. |
| Side effects | Clinically, benzalkonium chloride may cause eye irritation and is known to discolour soft contact lenses. Benzalkonium chloride in eye drops has also been reported to cause punctate keratopathy and/or toxic ulcerative keratopathy, especially in frequent or prolonged use or in conditions where the cornea is compromised. |
| Safety | is well tolerated in the dilutions normally employed on the skin and mucous membranes. However, benzalkonium chloride has been associated with adverse effects when used in some pharmaceutical formulations. Ototoxicity can occur when benzalkonium chloride is applied to the ear and prolonged contact with the skin can occasionally cause irritation and hypersensitivity. Benzalkonium chloride is also known to cause bronchoconstriction in some asthmatics when used in nebulizer solutions. Toxicity experiments with rabbits have shown benzalkonium chloride to be harmful to the eye in concentrations higher than that normally used as a preservative. However, the human eye appears to be less affected than the rabbit eye and many ophthalmic products have been formulated with benzalkonium chloride 0.01% w/v as the preservative. Benzalkonium chloride is not suitable for use as a preservative in solutions used for storing and washing hydrophilic soft contact lenses, as the benzalkonium chloride can bind to the lenses and may later produce ocular toxicity when the lenses are worn. Solutions stronger than 0.03% w/v concentration entering the eye require prompt medical attention. Local irritation of the throat, esophagus, stomach, and intestine can occur following contact with strong solutions (>0.1% w/v). The fatal oral dose of benzalkonium chloride in humans is estimated to be 1–3 g. Adverse effects following oral ingestion include vomiting, collapse, and coma. Toxic doses lead to paralysis of the respiratory muscles, dyspnea, and cyanosis. LD50 (mouse, oral): 150 mg/kg LD50 (rat, IP): 14.5 mg/kg LD50 (rat, IV): 13.9 mg/kg LD50 (rat, oral): 300 mg/kg LD50 (rat, skin): 1.42 g/kg |
| storage | Benzalkonium chloride is hygroscopic and may be affected by light, air, and metals. Solutions are stable over a wide pH and temperature range and may be sterilized by autoclaving without loss of effectiveness. Solutions may be stored for prolonged periods at room temperature. Dilute solutions stored in polyvinyl chloride or polyurethane foam containers may lose antimicrobial activity. The bulk material should be stored in an airtight container, protected from light and contact with metals, in a cool, dry place. |
| Incompatibilities | Incompatible with aluminum, anionic surfactants, citrates, cotton, fluorescein, hydrogen peroxide, hypromellose,iodides, kaolin, lanolin, nitrates, nonionic surfactants in high concentration, permanganates, protein, salicylates, silver salts, soaps, sulfonamides, tartrates, zinc oxide, zinc sulfate, some rubber mixes, and some plastic mixes. Benzalkonium chloride has been shown to be adsorbed to various filtering membranes, especially those that are hydrophobic or anionic. |
| Regulatory Status | Included in the FDA Inactive Ingredients Database (inhalations, IM injections, nasal, ophthalmic, otic, and topical preparations). Included in nonparenteral medicines licensed in the UK. It is also included in the Canadian List of Acceptable Non-medicinal Ingredients. |
| Benzalkonium chloride Preparation Products And Raw materials |
| Raw materials | Benzyl chloride |
| Preparation Products | Tetradecyldimethylbenzylammonium chloride |