| 4,6-Dichloropyrimidine Basic information |
| Uses |
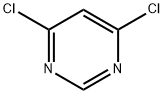
| Product Name: | 4,6-Dichloropyrimidine |
| Synonyms: | IFLAB-BB F2124-0077;PYRIMIDINE, 4,6-DICHLORO-;4 6-DICHLOROPYRIMIDINE 99%;4,6-Dichoropyrimidine;Pyrimidine, 4,6-dichloro- (7CI,8CI,9CI);4,6-DICHLOROPYRIMIDINE;4,6-Dichloropyrimidine ,98%;4,6-Dichloropyrimidine,97% |
| CAS: | 1193-21-1 |
| MF: | C4H2Cl2N2 |
| MW: | 148.98 |
| EINECS: | 214-770-2 |
| Product Categories: | Bases & Related Reagents;Nucleotides;Pyrazines, Pyrimidines & Pyridazines;Building Blocks;Halogenated Heterocycles;Heterocyclic Building Blocks;PyrimidinesHeterocyclic Building Blocks;Heterocycle-Pyrimidine series;alkyl chloride;Pyrimidines;Pyridines, Pyrimidines, Purines and Pteredines;PYRIMIDINE;FINE Chemical & INTERMEDIATES;Halides;Heterocycles;Pyrazines, Pyrimidines & Pyridazines;Nucleotides and Nucleosides;J’s;bc0001;1193-21-1 |
| Mol File: | 1193-21-1.mol |
 |
| 4,6-Dichloropyrimidine Chemical Properties |
| Melting point | 65-67 °C (lit.) |
| Boiling point | 176 °C (lit.) |
| density | 1.6445 (rough estimate) |
| vapor pressure | 30-390Pa at 20-50℃ |
| refractive index | 1.6300 (estimate) |
| Fp | 176°C |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | 95% ethanol: soluble50mg/mL, clear to very slightly hazy, colorless to yellow |
| pka | -4.20±0.17(Predicted) |
| form | Crystals |
| color | Yellow-green |
| BRN | 111195 |
| InChIKey | XJPZKYIHCLDXST-UHFFFAOYSA-N |
| LogP | 1.45 at 25℃ and pH7.2 |
| Surface tension | 65.72mN/m at 1g/L and 20℃ |
| CAS DataBase Reference | 1193-21-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Pyrimidine, 4,6-dichloro-(1193-21-1) |
| Safety Information |
| Hazard Codes | C |
| Risk Statements | 34-20/21/22-36/37 |
| Safety Statements | 26-36/37/39-45-27 |
| RIDADR | UN 3263 8/PG 2 |
| WGK Germany | 3 |
| F | 19 |
| Hazard Note | Corrosive |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 29335990 |
| Provider | Language |
|---|---|
| 4,6-Dichloropyrimidine | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| 4,6-Dichloropyrimidine Usage And Synthesis |
| Uses | 4,6-Dichloropyrimidine (cas# 1193-21-1) is a compound useful in organic synthesis. |
| Chemical Properties | Yellow Solid |
| Uses | 4,6-Dichloropyrimidine was used in the synthesis of N-substituted azacalix[4]pyrimidines. It was used as starting reagent for the synthesis of disubstituted pyrimidines by tandem amination and Suzuki-Miyaura cross-coupling. It was also used in a biarylpyrimidine synthesis involving biaryl cross-coupling. |
| General Description | Cyclic voltammograms of 4,6-dichloropyrimidine shows three cathodic waves, arising from sequential cleavage of carbon-chlorine bonds as well as the reduction of pyrimidine. |
| Synthesis | The synthesis of 4,6-Dichloropyrimidine is as follows:Dissolve 105.5g of 4,6-diaminopyrimidine in 660.0g of 31% hydrochloric acid and pour into a 2000ml bottle to cool to -5 ° C and dropwise add 500.3g of 33% sodium nitrite. After 2 hours of reaction, HPLC detects 4,6-diamino Pyrimidine is less than 0.5%. 42.8 g of cuprous chloride and 214.0 g of 31% hydrochloric acid are prepared in a 2000 ml bottle. The diazonium salt mother liquor is added dropwise to the bottle. After the dropwise reaction, the reaction is performed at 45 ° C for 2 hours. .Extract with 400g of recovered trichloroethane (200×2 times less than new ones), combine the organic layers for distillation, control the water flush pump 5KPa, temperature 40-140 , collect 404.2g of solvent in the early 40-90 , 90-140 in the later The product was collected at a temperature of 14 ° C to obtain 146.9 g of 4,6-dichloropyrimidine. The yield was 86.4% (based on formazan hydrochloride) and the purity was 99.4%.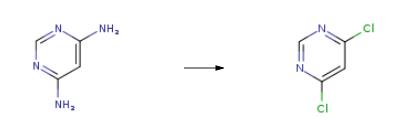 |
| 4,6-Dichloropyrimidine Preparation Products And Raw materials |