| 2,6-Dimethyl-5-heptenal Basic information |
| Product Name: | 2,6-Dimethyl-5-heptenal |
| Synonyms: | ,6-Dimethyl hept-5-en-1-al;2,6-dimethyl-5-heptena;2,6-dimethylhept-;2,6-dimethylhept-5-en-1-al;ai3-33278;nsc78450;Dimethylheptenal;2,6-Dimethyl-2-hepten-7-al |
| CAS: | 106-72-9 |
| MF: | C9H16O |
| MW: | 140.22 |
| EINECS: | 203-427-2 |
| Product Categories: | |
| Mol File: | 106-72-9.mol |
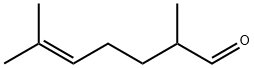 |
| 2,6-Dimethyl-5-heptenal Chemical Properties |
| Boiling point | 116-124 °C100 mm Hg(lit.) |
| density | 0.879 g/mL at 25 °C |
| vapor pressure | 2.39hPa at 25℃ |
| FEMA | 2389 | 2,6-DIMETHYL-5-HEPTENAL |
| refractive index | n20/D 1.444(lit.) |
| Fp | 141 °F |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| form | Liquid |
| Specific Gravity | 0.879 |
| color | Light orange to Yellow to Green |
| Odor | at 1.00 % in dipropylene glycol. fresh ozone melon fresh air sweet clean green |
| Odor Type | melon |
| Water Solubility | Soluble in alcohol, paraffin oil. Insoluble in water. |
| Sensitive | Air Sensitive |
| JECFA Number | 349 |
| InChIKey | YGFGZTXGYTUXBA-UHFFFAOYSA-N |
| LogP | 3.4 |
| CAS DataBase Reference | 106-72-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 5-Heptenal, 2,6-dimethyl-(106-72-9) |
| EPA Substance Registry System | 5-Heptenal, 2,6-dimethyl- (106-72-9) |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36/37/39 |
| RIDADR | 1987 |
| WGK Germany | 2 |
| RTECS | MJ8797000 |
| Hazard Note | Irritant |
| TSCA | Yes |
| HazardClass | 3 |
| PackingGroup | III |
| HS Code | 29121900 |
| toxicity | Both the acute oral LD50 value in rats and the acute dermal LD50 value in rabbits exceeded 5 g/kg (Levenstein, 1974). |
| Provider | Language |
|---|---|
| 2,6-Dimethyl-2-hepten-7-al | English |
| SigmaAldrich | English |
| ALFA | English |
| 2,6-Dimethyl-5-heptenal Usage And Synthesis |
| Chemical Properties | 2,6-Dimethyl-5-hepten-1-al was identified in ginger. It is a yellow liquid with a powerful, green, cucumber-like, and melon odor. It can be prepared by Darzens reaction of 6-methyl-5-hepten-2-one with ethyl chloroacetate. The intermediate glycidate is saponified and decarboxylated to yield the title compound. It is used in many fragrance types and is invaluable in the creation ofmelon and cucumber notes. |
| Chemical Properties | 2,6-Dimethyl-5-heptenal has a characteristic odor of melon and a corresponding taste. |
| Occurrence | Reported found in lemon and lime peel oil, sudachi (Citrus sudachi H. ex. Shirai) and ginger. |
| Uses | It is used or fragrances designed to have a special fruity note tending towards melon, cucumber and pumpkin. It is mainly used perfuming agents. |
| Preparation | By condensation of isobutyric aldehyde with α-methylcrotonaldehyde, followed by par tial hydrogenation; also from methylheptenone through Darzen’s reaction. |
| Aroma threshold values | Detection: 16 ppb |
| Taste threshold values | Taste characteristics at 50 ppm: green, melon, watermelon-rind, cucumber, with a waxy, chemical and floral nuance. |
| General Description | 2,6-Dimethyl-5-heptenal (melonal) and 5-heptenal undergo concerted ene reaction to form zwitterion that further reacts to give ene product. |
| Flammability and Explosibility | Notclassified |
| Trade name | Melonal (Givaudan), Melomor (Aromor). |
| Safety Profile | skin and eye irritant. When heated to decomposition it emits acrid smoke and irritating fumes. |
| 2,6-Dimethyl-5-heptenal Preparation Products And Raw materials |
| Raw materials | Isobutyraldehyde–>Crotonaldehyde |
| Preparation Products | METHOPRENE |