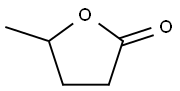
| Description | γ-Valerolactone (Item No. 28240) is an analytical reference standard categorized as a prodrug form of γ-hydroxyvaleric acid (GHV; ). This product is intended for research and forensic applications. |
| Chemical Properties | Colorless liquid. Surface tension 30 dynes/cm (25C), viscosity 2.18 cP (25C), pH (anhydrous): 7. pH (10% solution in distilled water): 4.2. Miscible with water and most organic solvents, resins, waxes, etc.; slightly misciblewith zein, beeswax, petrolatum; immiscible with anhydrous glycerin, glue, casein, arabic gum, and soybean protein. Combustible. |
| Chemical Properties | γ-Valerolactone has a sweet, herbaceous odor. |
| Occurrence | Reported found in boiled beef, beef fat, beer, cacao, Swiss cheese, ground and roasted coffee, roasted filberts, milk fat, dried mushroom, peach, roasted peanuts, heated pork fat, black tea and yogurt. Also reported found in peach, strawberry jam, tomato, wheaten bread, Gruyere cheese, heated butter, cooked beef, white wine, red wine, coffee and Bourbon vanilla. |
| Uses | It finds it application as a food additive that is used to improve the taste or odor of a food. It is also used as toiletry fragrances. |
| Uses | γ-valerolactone?(GVL) can be used as a green solvent:
To transform lignocellulose into furfural using a solid acid catalyst, H-mordenite.To synthesize phosphatidylserine. |
| Uses | γ-Valerolactone is a naturally occurring chemical found in fruits and is frequently used as a food additive. It can be converted to liquid alkenes which can be used as transportation fuels. γ-Valerolactone is widely used in dye baths (coupling agent), brake fluids, cutting oils, and as solvent for adhesives, insecticides, and lacquers. |
| Definition | ChEBI: Gamma-valerolactone is a butan-4-olide that is dihydrofuran-2(3H)-one substituted by a methyl group at position 5. It has been found in the urine samples of humans exposed to n-hexane. It has a role as a flavouring agent and a human xenobiotic metabolite. |
| Preparation | By reduction of levulinic acid followed by cyclization. |
| Synthesis Reference(s) | Journal of the American Chemical Society, 112, p. 1286, 1990 DOI: 10.1021/ja00159a082
Tetrahedron Letters, 26, p. 5639, 1985 DOI: 10.1016/S0040-4039(01)80907-2
The Journal of Organic Chemistry, 50, p. 3930, 1985 DOI: 10.1021/jo00220a053 |
| General Description | γ-Valerolactone has been identified as one of the volatile flavor constituents in mango and honey. |
| Reactivity Profile | gamma-Valerolactone is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. gamma-Valerolactone is incompatible with strong oxidizers. . gamma-Valerolactone is incompatible with strong oxidizing agents. gamma-Valerolactone is also incompatible with strong acids, strong bases and strong reducing agents. . |
| Biochem/physiol Actions | Odor at 1% |
| Safety Profile | Moderately toxic by ingestion. A skin irritant. Mutation data reported. Combustible liquid when exposed to heat or flame; can react with oxidizing materials. To fight fire, use water, foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Purification Methods | Purify the -lactone by repeated fractional distillation [Boorman & Linstead J Chem Soc 577, 580 1933]. IR: max 1790 (CS2), 1775 (CHCl3) cm-1 [Jones et al. Can J Chem 3 7 2007 1959]. The BF3-complex distils at 110-111o/20mm [Reppe et al. Justus Liebigs Ann Chem 596 179 1955]. It is characterized by conversion to -hydroxy-n-valeramide on treatment with NH3, m 51.5-52o (by slow evaporation of a CHCl3 solution). [Beilstein 17 H 235, 17 I 131, 17 II 288, 17 III/IV 4176, 17/9 V 24.] |