| Vardenafil Basic information |
| Product Name: | Vardenafil |
| Synonyms: | VARDENAFIL(SUBJECTTOPATENTFREE);VARDENAFILHYDROCHLORIDETRIHYDRATE(SUBJECTTOPATENTFREE);2-(2-Ethoxy-5-(4-ethylpiperazin-1-yl-1-sulfonyl)phenyl)-5-methyl-7-propyl-3H-imidazo(5,1-f)(1,2,4)triazin-4-one;1-[[3-(1,4-Dihydro-5-methyl-4-oxo-7-propylimidazo[5,1-f][1,2,4]triazin-2-yl)-4-ethoxyphenyl]sulfonyl]-4-ethyl-piperazine hydrochloride trihydrate;2-[2-ethoxy-5-(4-ethylpiperazin-1-yl)sulfonyl-phenyl]-5-methyl-7-propyl-1H-imidazo[5,1-f][1,2,4]triazin-4-one;2-[2-ethoxy-5-(4-ethylpiperazin-1-yl)sulfonylphenyl]-5-methyl-7-propyl-1H-imidazo[5,1-f][1,2,4]triazin-4-one;Methanol (test Vardenafil diHCl, 1.0 mg/mL as free base);Vardenafil (Vivanza) |
| CAS: | 224785-90-4 |
| MF: | C23H32N6O4S |
| MW: | 488.6 |
| EINECS: | 607-088-5 |
| Product Categories: | API;Levitra;Inhibitor;Erectile Dysfunction |
| Mol File: | 224785-90-4.mol |
 |
| Vardenafil Chemical Properties |
| Melting point | 230-235°C |
| density | 1.37 |
| vapor pressure | 0.001Pa at 71.34℃ |
| Fp | 9℃ |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | >22.5mg/mL in DMSO |
| form | Powder |
| pka | 9.86±0.20(Predicted) |
| Water Solubility | 7mg/L at 20℃ |
| BCS Class | 2 |
| InChIKey | NOIHTGOGFDFCBN-UHFFFAOYSA-N |
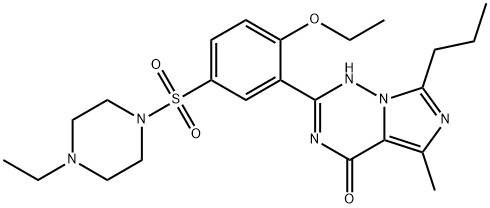
| SMILES | N12C(CCC)=NC(C)=C1C(=O)N=C(C1=CC(S(N3CCN(CC)CC3)(=O)=O)=CC=C1OCC)N2 |
| LogP | 2.5 at 23℃ |
| Safety Information |
| Hazard Codes | F,Xn |
| Risk Statements | 11-20/21/22-36 |
| Safety Statements | 16-36/37 |
| RIDADR | UN1230 – class 3 – PG 2 – Methanol, solution |
| WGK Germany | 1 |
| Hazardous Substances Data | 224785-90-4(Hazardous Substances Data) |
| Vardenafil Usage And Synthesis |
| Description | Vardenafil is a new PDE5 inhibitor launched for oral treatment of male erectile dysfunction and it has significant structural similarity with sildenafil (Viagra?), which was the first PDE5 inhibitor introduced in 1998 for this indication. Vardenafil is synthesized in three steps starting with a cyclization reaction of 2-ethyoxybenzamidine with 2-butyramidopropionic acid and ethoxyallyl chloride to construct the imidazotriazine ring system, followed by sulfonation to the corresponding sulfonyl chloride and subsequent condensation with 1-ethylpiperazine. The potency of PDE5 inhibition by vardenafil (IC50=0.7 nM) is ~10 times greater than that of sildenafil (IC50=6.6 nM). Vardenafil is typically administered in single doses of 10 and 20 mg. The time to reach maximum plasma concentration is 0.75 h, which is slightly shorter than those of sildenafil (tmax=1.16 h) and tadalafil (tmax=2h), and the half-life is 4–5 h. Although it is almost completely absorbed following oral administration, the mean absolute bioavailability of a 10 mg dose is ~15%, resulting from extensive first pass metabolism. Vardenafil is metabolized in the liver primarily by CYP3A4 and is eliminated mainly in feces. In clinical studies, 10–20 mg doses of vardenafil was well tolerated and efficacious in patients with ED of various severities, including subjects with comorbidities such as diabetes mellitus or hypertension or hyperlipidemia. The side-effect profile of vardenafil is similar to that of sildenafil, with headache, flushing, dyspepsia and nasal congestion being the most common adverse events. Vardenafil has systemic vasodilatory properties, which can cause transient decrease in supine blood pressure; however, it does not appear to translate into clinical effects. The mean maximum decreases in supine systolic blood pressure following 20 and 40 mg vardenafil were 6.9 and 4.3 mmHg, respectively, when compared to placebo. However, single and multiple oral doses of vardenafil up to 40 mg produced no clinically relevant changes in the ECGs of normal male volunteers. |
| Originator | Bayer AG (Germany) |
| Uses | erectil dysfunction;PDE5 inhibitor |
| Uses | Vardenafil Hydrochloride Trihydrate (cas# 330808-88-3) has therapeutic applications and used in cosmetics and personal care products. |
| Definition | ChEBI: The sulfonamide resulting from formal condensation of the sulfo group of 4-ethoxy-3-(5-methyl-7-propylimidazo[5,1-f][1,2,4]triazin-4(1H)-one-2-yl)benzenesulfonic acid and the secondary amino group of 4-ethylpiperazine. |
| Brand name | Levitra |
| Flammability and Explosibility | Nonflammable |
| Clinical Use | Treatment of erectile dysfunction |
| Drug interactions | Potentially hazardous interactions with other drugs Alpha-blockers: enhanced hypotensive effect – avoid for 6 hours after alpha-blockers (max dose 5 mg). Antifungals: concentration increased by ketoconazole, and itraconazole – avoid concomitant use. Antivirals: concentration increased by fosamprenavir, indinavir and ritonavir- avoid with indinavir and ritonavir; increased risk of ventricular arrhythmias with saquinavir – avoid; avoid with telaprevir, use tipranavir with caution. Cobicistat: concentration of vardenafil possibly increased – reduce dose of vardenafil. Grapefruit juice: concentration possibly increased – avoid concomitant use. Nicorandil: possibly enhanced hypotensive effect – avoid concomitant use. Nitrates: possibly enhanced hypotensive effect – avoid concomitant use. Riociguat: enhanced hypotensive effect – avoid concomitant use. |
| Metabolism | Vardenafil is metabolised in the liver primarily by cytochrome P450 isoenzymes CYP3A4 (the major route) as well as CYP3A5 and CYP2C isoforms. The major metabolite produced by desethylation of vardenafil also has some activity. Vardenafil is excreted as metabolites mainly in the faeces (91 to 95 %), and to a lesser extent in the urine. |
| Vardenafil Preparation Products And Raw materials |