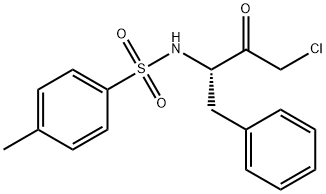
| Description | TPCK (402-71-1) is an irreversible inhibitor of the serine protease chymotrypsin.1 It also inhibits certain cysteine proteases (bromelain, ficin, papain).1 TPCK inhibited (IC50 = 5 μM) the mitogen-induced activation of pp70s6k, a mitogen-regulated serine/threonine kinase involved in the G1 to S phase transition of the cell cycle.2 |
| Chemical Properties | white crystalline powder |
| Uses | N-α-Tosyl-L-phenylalanylchloromethane is a proteinase inhibitors with apoptotic function. Studies have shown that it induces caspase-dependent apoptosis in Epstein-Barr virus (EBV)-transformed human B cell lines with release of pro-apoptotic proteins from mitochondria. It also results in down-regulation of the anti-apoptotic proteins and caspase-dependent cleavage of two anti-apoptotic proteins. I t promotes dephosphorylation of p53 on serine residues. |
| Uses | N-α-Tosyl-L-phenylalanylchloromethane is a proteinase inhibitors with apoptotic function. Studies have shown that it induces caspase-dependent apoptosis in Epstein-Barr virus (EBV)-transformed human B cell lines with release of pro-apoptotic proteins from mitochondria. It also results in down-regulation of the anti-apoptotic proteins and caspase-dependent cleavage of two anti-apoptotic proteins. It promotes dephosphorylation of p53 on serine residues. |
| Definition | ChEBI: The N-tosyl derivative of L-phenylalanyl chloromethyl ketone. |
| Biochem/physiol Actions | Blocks the LPS- or cytokine-induced activation of nuclear factor κB (NFκB), which, in turn, blocks the induction of iNOS and COX-2 transcription. Blocks activation of pp70s6k by all mitogens. Blocks apoptosis cell lines by inhibiting the processing of caspases in some cell lines and to some stimuli. Also blocks apoptosis initiated by Taxol (in MCF-7 human breast carcinoma cells). |
| Safety Profile | Experimental reproductive effects. A flammable liquid. When heated to decomposition it emits toxic fumes of NOx, SOx, and Cl-. |
| References | 1) Bond and Butler, (1987) Intracellular proteases; Annu. Rev. Biochem., 56 333 2) Grammer and Blenis (1996) The serine protease inhibitors, tosylphenylalanine chloromethylketone and tosyllysine chloromethylketone, potently inhibit pp70s6k activation; J. Biol. Chem., 271 23650 |