| Shikimic acid Basic information |
| Product Name: | Shikimic acid |
| Synonyms: | Shikimic acid,(3R,4S,5R)-()-3,4,5-Trihydroxy-1-cyclohexenecarboxylic acid;Illicium Verum P.E.;Shikimic acid methyl esther;ShikiMic acid, 98% 1GR;3,4,5-Trihydroxycyclohex-1-enecarboxylic acid;Shikimic acid,98%;ShikiMic acid API;ShikiMic acid (ShikiMate) |
| CAS: | 138-59-0 |
| MF: | C7H10O5 |
| MW: | 174.15 |
| EINECS: | 205-334-2 |
| Product Categories: | Inhibitors;Plant Extract;chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;API;Antibiotic;Chiral Building Blocks;Simple Alcohols (Chiral);Synthetic Organic Chemistry;Natural Plant Extract;Chiral Reagents;API intermediates;138-59-0 |
| Mol File: | 138-59-0.mol |
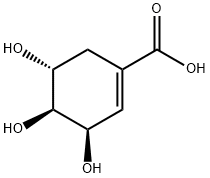 |
| Shikimic acid Chemical Properties |
| Melting point | 185-187 °C (lit.) |
| Boiling point | 225.11°C (rough estimate) |
| alpha | -180 º (c=4, H2O 25 ºC) |
| density | 1.52 g/cm3 (27.2℃) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | -180 ° (C=1, H2O) |
| storage temp. | 2-8°C |
| solubility | 180g/l |
| form | Powder |
| pka | pK (14.1°) 5.19 |
| color | White to light beige or light gray |
| optical activity | [α]/D -180.0±5.0°, c = 4 in H2O |
| Water Solubility | 18 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8480 |
| BRN | 4782717 |
| InChIKey | JXOHGGNKMLTUBP-HSUXUTPPSA-N |
| LogP | -1.5 at 21℃ and pH1 |
| Surface tension | 49.86mN/m at 1g/L and 20℃ |
| CAS DataBase Reference | 138-59-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Shikimic acid(138-59-0) |
| IARC | 3 (Vol. 40, Sup 7) 1987 |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 22-24/25-36-26 |
| WGK Germany | 3 |
| RTECS | GW4600000 |
| F | 3-10 |
| HS Code | 29181980 |
| Hazardous Substances Data | 138-59-0(Hazardous Substances Data) |
| Toxicity | LD5 i.p. in mice: 1000 mg/kg (Evans, Osman) |
| Provider | Language |
|---|---|
| Shikimic acid | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Shikimic acid Usage And Synthesis |
| Chemical Properties | White Solid |
| Uses | Shikimic acid has been used as a standard for the quantification of shikimate in apical parts of roots. It has also been used as a substrate in shikimate kinase assay. |
| Uses | Naturally occurring (-)-form is a major biosynthetic precursor of phenylalanine, tyrosine, and tryptophan and hence of the majority of plant alkaloids. It is also involved in the biosynthesis of lignin, flavonpids and other important aromatic compounds. |
| Definition | ChEBI: A cyclohexenecarboxylic acid that is cyclohex-1-ene-1-carboxylic acid substituted by hydroxy groups at positions 3, 4 and 5 (the 3R,4S,5R stereoisomer). It is an intermediate metabolite in plants and micro rganisms. |
| Biochem/physiol Actions | Shikimic acid is observed to be carcinogenic in rat models, when administered. It serves as a precursor for the biosynthesis of aromatic amino acids, alkaloids and other aromatic metabolites in microorganisms. Shikimic acid is known to inhibit adenosine diphosphate (ADP) induced platelet aggregation and blood coagulation in rabbits. |
| Shikimic acid Preparation Products And Raw materials |