| m-Nitrobenzoyl chloride Basic information |
| Product Name: | m-Nitrobenzoyl chloride |
| Synonyms: | LABOTEST-BB LTBB000612;M-NITROBENZOYL CHLORIDE;m-Nitrobenzoylchroride;META NITRO BENZOYL CHLORIDE;3-NITROBENZOYL CHLORIDE 99+%;3-Nitrobenzoyl chloride,98%;m-Nitrobenzoyl chlor;3-Nitrobenzoyl chloride, 98% 100GR |
| CAS: | 121-90-4 |
| MF: | C7H4ClNO3 |
| MW: | 185.56 |
| EINECS: | 204-505-9 |
| Product Categories: | Acid Halides;Building Blocks;Carbonyl Compounds;Chemical Synthesis;Organic Building Blocks;Acid Halides;Carbonyl Compounds;Organic Building Blocks |
| Mol File: | 121-90-4.mol |
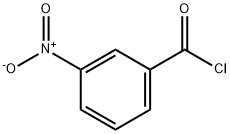 |
| m-Nitrobenzoyl chloride Chemical Properties |
| Melting point | 31-34 °C(lit.) |
| Boiling point | 275-278 °C(lit.) |
| density | 1.42 |
| refractive index | 1.5810 (estimate) |
| Fp | >230 °F |
| solubility | soluble in Ether,Benzene,Toluene |
| form | Low Melting Solid |
| Specific Gravity | 1.59 |
| color | Brown |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| BRN | 777186 |
| CAS DataBase Reference | 121-90-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzoyl chloride, 3-nitro-(121-90-4) |
| EPA Substance Registry System | m-Nitrobenzoyl chloride (121-90-4) |
| Safety Information |
| Hazard Codes | C |
| Risk Statements | 5-21-34-37 |
| Safety Statements | 26-36/37/39-45-7/9-38 |
| RIDADR | UN 2923 8/PG 3 |
| WGK Germany | 3 |
| RTECS | DM6646000 |
| F | 9-19-21 |
| Hazard Note | Corrosive |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 29163900 |
| Provider | Language |
|---|---|
| m-Nitrobenzoyl chloride | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| m-Nitrobenzoyl chloride Usage And Synthesis |
| Chemical Properties | BROWN LOW MELTING SOLID |
| Uses | 3-Nitrobenzoyl Chloride is an useful building block reagent in the preparation of organic compounds of research interest. |
| Uses | Manufacture of dyes for fabrics and color photography, intermediate in preparation of pharmaceuticals. |
| Uses | 3-Nitrobenzoyl chloride was used in the synthesis of 3-aminobenzamide. It was also used to synthesize potential carbocyclic minor groove binders. |
| General Description | Yellow to brown liquid. Unstable at room temperatures; requires refrigeration when not in use. |
| Air & Water Reactions | May be unstable to prolonged exposure to air. Decomposes in water and alcohol. . |
| Reactivity Profile | m-Nitrobenzoyl chloride is incompatible with bases (including amines), with strong oxidizing agents, and with alcohols. May react with reducing agents. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. |
| Hazard | Toxic by ingestion. |
| Health Hazard | ACUTE/CHRONIC HAZARDS: m-Nitrobenzoyl chloride is unstable at room temperature or when exposed to sources of ignition. When heated to decomposition it emits highly toxic fumes. It will react with water or steam to produce toxic and corrosive fumes. |
| Fire Hazard | m-Nitrobenzoyl chloride is combustible. |
| m-Nitrobenzoyl chloride Preparation Products And Raw materials |
| Raw materials | Thionyl chloride–>Benzene–>3-Nitrobenzoic acid–>2-Nitrobenzoyl chloride–>Benzoyl chloride |
| Preparation Products | 3-Nitrobenzaldehyde–>3-NITROBENZHYDRAZIDE–>3-Nitrobenzyl alcohol–>3-AMINO-N,N-DIMETHYLBENZYLAMINE |