| Description | (−)-Epicatechin is a polyketide synthase-derived polyphenol flavonoid that has been found in T. cacao and has diverse biological activities. It scavenges DPPH radicals in a cell-free assay when used at a concentration of 5 μM. (−)-Epicatechin inhibits COX-1 (IC50 = 3.2 μM). It acts synergistically with epigallocatechin gallate to induce apoptosis in, and reduce the proliferation of, PC-9 lung cancer cells when used at a concentration of 200 μM. (−)-Epicatechin (80 mg/kg) reduces LPS-induced increases in plasma creatinine and urea levels in a rat model of renal inflammation. |
| Chemical Properties | white to light yellow crystal powde |
| Uses | An antioxidant and natural product from green tea |
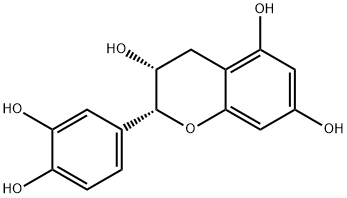
| Uses | Catechin is a polyphenolic flavonoid that has been isolated from a variety of natural sources including tea leaves, grape seeds, and the wood and bark of trees such as acacia and mahogany. Catechin is a more potent antioxidant than ascorbate or α-tocopherol in certain in vitro lipid peroxidation assays. (?)-Epicatechin is a 2R,3R stereoisomer of catechin. Like catechin, (?)-epicatechin is a powerful antioxidant. It inhibits cyclooxygenase 1 (IC50 = 3.2 μM). Also, at 100 μM, (?)-epicatechin induces apoptosis in human adenocarcinoma PC-9 cells and stimulates the inhibition of tumor necrosis factor-α release from BALB-c/3T3 cells treated with epigallocatechin gallate. |
| Uses | Potent antioxidant and antineoplastic agent. |
| Definition | ChEBI: A catechin with (2R,3R)-configuration. |
| General Description | (?)-Epicatechin (EC) belongs to the group of flavanols and is abundantly present in cacao and cacao products. The chemical structure of EC includes an oxygenated heterocycle with a 4-hydroxyl group linked with two aromatic rings. |
| Biochem/physiol Actions | This antioxidant is found in chocolate. (-)-Epicatechin is linked with cardiovascular effects. It is considered as a dependable standard due to its availability in cacao seeds and cocoa products. The halogenating activity of myeloperoxidase can be restored by (-)-epicatechin. |
| references | [1]. chakravarthy bk, gupta s, gambhir ss, et al. pancreatic beta-cell regeneration in rats by (-)-epicatechin. lancet, 1981, 2(8249): 759-760.
[2]. wippel r, rehn m, gorren ac, et al. interference of the polyphenol epicatechin with the biological chemistry of nitric oxide- and peroxynitrite-mediated reactions. biochem pharmacol, 2004, 67(7): 1285-1295.
[3]. brossette t, hundsdörfer c, kröncke kd, et al. direct evidence that (-)-epicatechin increases nitric oxide levels in human endothelial cells. eur j nutr, 2011, 50(7): 595-599.
[4]. okushio k, suzuki m, matsumoto n, et al. identification of (-)-epicatechin metabolites and their metabolic fate in the rat. drug metab dispos, 1999, 27(2): 309-316. |