| Fexofenadine hydrochloride Basic information |
| Description Mechanism of Action Biochem/physiol Actions Indications Overdosage References |
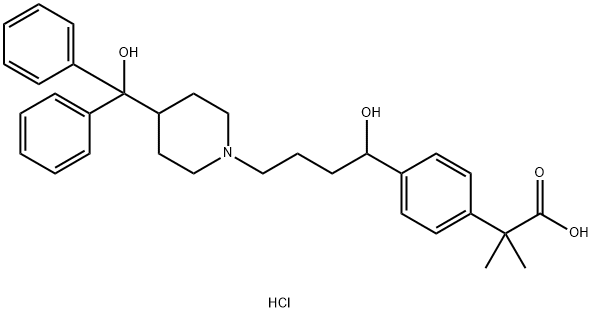
| Product Name: | Fexofenadine hydrochloride |
| Synonyms: | α,α-dimethyl-4-[1-hydroxy-4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]butyl]benzeneacetic acid;Benzeneacetic acid, 4-[1-hydroxy-4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]butyl]-α,α-dimethyl-, hydrochloride;MDL 16455A;Telfast;Telfast BD;Fexofenadine hydrochloride;2-[4-[1-hydroxy-4-[4-[hydroxy(diphenyl)methyl]-1-piperidyl]butyl]phenyl]-2-methyl-propionic acid hydrochloride;FERULICACIDPIPERAZINE |
| CAS: | 153439-40-8 |
| MF: | C32H40ClNO4 |
| MW: | 538.13 |
| EINECS: | 604-906-2 |
| Product Categories: | API;Aromatics;Heterocycles;Metabolites & Impurities;Intermediates & Fine Chemicals;Metabolites;Pharmaceuticals;153439-40-8 |
| Mol File: | 153439-40-8.mol |
 |
| Fexofenadine hydrochloride Chemical Properties |
| Melting point | 148-150oC |
| RTECS | CY1633377 |
| storage temp. | 2-8°C |
| solubility | Soluble in Dimethyl sulfoxide (50 mM), and methanol. |
| form | powder |
| color | white to beige |
| Merck | 14,4068 |
| InChIKey | RRJFVPUCXDGFJB-UHFFFAOYSA-N |
| CAS DataBase Reference | 153439-40-8(CAS DataBase Reference) |
| Safety Information |
| Fexofenadine hydrochloride Usage And Synthesis |
| Description | Fexofenadine hydrochloride (trade name: Allegra) belongs to a second-generation antihistamine pharmaceutical drug. It is mainly used for the treatment of allergy symptoms including watery eyes, hay fever, nasal congestion, urticaria, runny nose, itching eyes/nose, sneezing, hives, and itching. It is a kind of selectively peripheral H1 blocker. Its mechanism of action in vivo is through preventing the histamine-mediated activation of the H1 receptors, thus alleviating the symptoms related to allergy. Compared to the first-generation antihistamine drug, it is less prone to penetrate the blood-brain barrier, reducing the possibility to cause side effects such as drowsiness. |
| Mechanism of Action | Fexofenadine hydrochloride is an antihistamine with selective peripheral H1-receptor antagonist activity. Both enantiomers of fexofenadine hydrochloride displayed approximately equipotent antihistaminic effects. Fexofenadine inhibited histamine release from peritoneal mast cells in rats. In laboratory animals, no anticholinergic, alpha1-adrenergic or beta-adrenergic-receptor blocking effects were observed. No sedative or other central nervous system effects were observed. Radiolabeled tissue distribution studies in rats indicated that fexofenadine does not cross the blood-brain barrier. |
| Biochem/physiol Actions | Fexofenadine is used in treating allergic rhinitis and chronic idiopathic urticaria. It may give relief in bothersome symptom and is a second-generation antihistamines. Fexofenadine provides better relief compared to other antihistamines for nasal congestion. It is the active metabolite of terfenadine and bring down allergen-induced symptoms. Fexofenadine is a non-sedating H1 histamine receptor antagonist. |
| Indications | Seasonal Allergic Rhinitis Fexofenadine hydrochloride(ALLEGRA) is indicated for the relief of symptoms associated with seasonal allergic rhinitis in adults and children 6 years of age and older. Symptoms treated effectively were sneezing, rhinorrhea, itchy nose/palate/throat, itchy/watery/red eyes. Chronic Idiopathic Urticaria ALLEGRA is indicated for treatment of uncomplicated skin manifestations of chronic idiopathic urticaria in adults and children 6 years of age and older. It significantly reduces pruritus and the number of wheals. CONTRAINDICATIONS ALLEGRA is contraindicated in patients with known hypersensitivity to any of its ingredients. |
| Overdosage | Reports of fexofenadine hydrochloride overdose have been infrequent and contain limited information. However, dizziness, drowsiness, and dry mouth have been reported. Single doses of fexofenadine hydrochloride up to 800 mg (six normal volunteers at this dose level), and doses up to 690 mg twice daily for 1 month (three normal volunteers at this dose level) or 240 mg once daily for 1 year (234 normal volunteers at this dose level) were administered without the development of clinically significant adverse events as compared to placebo. In the event of overdose, consider standard measures to remove any unabsorbed drug. Symptomatic and supportive treatment is recommended. Hemodialysis did not effectively remove fexofenadine hydrochloride from blood (1.7% removed) following terfenadine administration. No deaths occurred at oral doses of fexofenadine hydrochloride up to 5000 mg/kg in mice (110 times the maximum recommended daily oral dose in adults and 200 times the maximum recommended daily oral dose in children based on mg/m2 ) and up to 5000 mg/kg in rats (230 times the maximum recommended daily oral dose in adults and 400 times the maximum recommended daily oral dose in children based on mg/m2 ). Additionally, no clinical signs of toxicity or gross pathological findings were observed. In dogs, no evidence of toxicity was observed at oral doses up to 2000 mg/kg (300 times the maximum recommended daily oral dose in adults and 530 times the maximum recommended daily oral dose in children based on mg/m2 ). |
| References | http://www.rxlist.com/allegra-drug/consumer-uses.htm https://en.wikipedia.org/wiki/Fexofenadine |
| Description | Fexofenadine is a histamine H1 receptor antagonist (Ki = 10 nM). It reverses contraction of isolated rat tracheal strips induced by acetyl-β-methylcholine . Fexofenadine inhibits expression of IL-8 in TNF-α-stimulated HCT116 and COLO 205 cells. Oral administration of fexofenadine (2 and 10 mg/kg) reduces severity of colitis and phospho-IκB kinase activation in a mouse model of colitis induced by dextran sulfate sodium (DSS; ). |
| Chemical Properties | Off-White Crystalline Solid |
| Originator | Alernex,Dabur Pharmaceuticals Ltd.,India |
| Uses | Fexofenadine is a non-sedating antihistamine that selectively antagonizes the histamine H1 receptor with a Ki value of 10 nM and exhibits anti-inflammatory effects. It is devoid of central nervous system effects in part because it is a good substrate for the P-glycoprotein efflux pump situated within the blood-brain barrier.[Cayman Chemical] |
| Uses | Antihistaminic;histamine H1-receptor antagonist |
| Uses | A metabolite of of terfenadine, a H1-Histamine receptor antagonist |
| Uses | The active metabolite of Terfenadine (T114500), a H1-histamine receptor antagonist. |
| Uses | Fexofenadine hydrochloride is used as an antihistamine that inhibits Cox-1, Cox-2, and is a histamine H1 receptor agonist. |
| Definition | ChEBI: Fexofenadine hydrochloride is a diarylmethane. |
| Manufacturing Process | Microbiological Preparation of Fexofenadine (Patent U.S. 6,558,931) Ten 250 ml Erlenmeyer flasks containing 100 ml of medium D are seeded with A. corymbifera LCP 63-1800. 50 mg of Terfenadine in 1 ml of ethanol is added to each Erlenmeyer flask. The content of 10 Erlenmeyer flasks are filtered on gauze (the supernatant has a pH equal to 8.0), and saturation with sodium chloride is carried out for 2 hours (pH = 5-6), followed by extracting 3 times with ethyl acetate and drying over magnesium sulfate. After evaporation under reduced pressure, 409 mg of expected crude product is obtained (yield = 77%, approximately 90% pure product). This product is purified on a column of silica gel (230-400 mesh, 40 g of silica, diameter = 3 cm) eluting with a methylene chloride/methanol/ammonium hydroxide mixture (82.5:15:2.5). 312 mg of pure Fexofenadine is recovered (61.4%). Synthesis of Fexofenadine (Patent U.S. No. 5,578,610 Aluminum chloride (44 g; 0.33 mol) was added in portions to a solution of freshly distilled 4-chlorobutyryl chloride (17 mL; 0.15 mol) in 460 mL of carbon disulfide at -10°C under a nitrogen atmosphere. The mixture was stirred for 15 min, then the cooling bath was removed and the mixture was allowed to warm to ambient temperature. The mixture was stirred then for 15 min more, then cooled again to -10°C and a solution of ethyl-α,α- dimethylphenyl acetate (26.6 g; 0.14 mol) in 70 mL of carbon disulfide was added dropwise. The mixture was stirred for 3 hours, then stirred overnight at room temperature. The reaction mixture was partitioned between water and CHCl3. The combined organic portions were dried over MgSO4, filtered and concentrated in vacuo. The residue was dissolved in CH2Cl2 and filtered through a plug of SiO2, eluting with 10% EtOAc in hexane. Yield of ethyl 3- and 4-(4-chloro-1-oxobutyl)-α,α-dimethylphenylacetate 39.4 g (as a mixture of aromatic regioisomers). To a solution of 39.4 g of ethyl 3- and 4-(4-chloro-1-oxobutyl)-α,α- dimethylphenylacetate dissolved in 800 mL of methanol and 200 mL of water was added 40 g of NaOH. The mixture was refluxed for one hour. The cooled mixture was then concentrated in vacuo. The concentrate was diluted with water and washed with 2 portions of EtOAc. The aqueous layer was acidified with concentrated HCl and extracted with 2 portions of EtOAc. The extracts were dried over MgSO4, filtered, and concentrated in vacuo to afford 30.3 g of crude product. The crude product was dissolved in 600 mL of EtOAc, 38 g of cinchonidine was added, and the mixture was stirred overnight. The resulting solids were filtered and washed with EtOAc and sucked dry under a rubber dam to afford 25 g of a solid 4-(cyclopropyl-oxo-methyl)-α,α- dimethylphenylacetic acid. A solution of 10.5 g of 4-(cyclopropyl-oxo-methyl)-α,α-dimethylphenylacetic acid in 250 mL of CH2Cl2 was cooled in an ice-MeOH bath and 25 g of trimethylsilyliodide was then added via pipette. The mixture was stirred in the ice bath for one hour, warmed to ambient temperature, and stirred for one hour. A solution of aqueous sodium bisulfite was then added and the mixture was stirred well. The phases were partitioned and the aqueous layer was extracted with CH2Cl2. The combined organics were washed with saturated aqueous NaCl, dried over MgSO4, filtered, and concentrated in vacuo to afford 12.6 g (77%) of 4-(4-iodo-1-oxobutyl)-α,α-dimethylphenylacetic acid. To a solution of 12.6 g of 4-(4-iodo-1-oxobutyl)-α,α-dimethylphenylacetic acid in 100 mL of ether cooled in an ice bath, was added 40 mL of ethereal CH2N2. The mixture was stirred at 0°C for few minutes, then let stand for 2 hours. A few drops of AcOH were added to decompose excess CH2N2, then the mixture was filtered and stripped to afford 12.6 g (96%) of methyl 4-(4-iodo-1- oxobutyl)-α,α-dimethylphenylacetate. A solution of 12.6 g of methyl 4-(4-iodo-1-oxobutyl)-α,α- dimethylphenylacetate in 500 mL of toluene in a one liter three neck flask was added 8.8 g of 4-(α,α-diphenyl)piperidinemethanol and 23 g of K2CO3 and the mixture was refluxed for 7 hours. The cooled reaction mixture was then filtered and concentrated in vacuo. The residue was dissolved in ether and treated with excess ethereal HCl. The mixture was then concentrated to a solid. The solid was treated with EtOAc and collected by filtration. The product was then partitioned between EtOAc and 2 N Na2CO3. The organics were dried over MgSO4, filtered, and concentrated in vacuo to afford 13.5 g (79%) of methyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-oxobutyl]-α,α- dimethylphenylacetate. A solution of 13.5 g of methyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]- 1-oxobutyl]-α,α-dimethylphenylacetate in 250 mL of methanol was cooled in an ice-methanol bath and 1.8 g of NaBH4 was added in portions. After 1 hour, the mixture was concentrated to a solid. The residue was partitioned between EtOAc and saturated aqueous NaHCO3. The aqueous portion was extracted with EtOAc. The combined organics were washed with saturated aqueous NaCl, dried over MgSO4, filtered, and concentrated in vacuo to afford 9.5 g (70%) of methyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1- hydroxybutyl]-α,α-dimethylphenylacetate as a foam. To a solution of 9.5 g of methyl-4-[4-[4-(hydroxydiphenylmethyl)-1- piperidinyl]-1-hydroxybutyl]-α,α-dimethylphenylacetate in 300 mL of methanol and 150 mL of water was added 10 g of NaOH. The mixture was refluxed for 1 hour, then cooled. The methanol was removed in vacuo. The concentrate was diluted with water and CHCl3 and the pH adjusted to approximately 5.5 to 6.0. The phases were separated and the aqueous phase was extracted with CHCl3. The combined organics were dried over MgSO4, filtered, and stripped to afford 9.0 g of crude product. The crude product was dissolved in CH2Cl2 and chromatographed on Davisil Grade 633 SiO2 eluting with a gradient of CHCl3, to 10% of methanol in CHCl3, to 25% of methanol in CHCl3. The product was concentrated to afford 5.2 g of white crystals of 4-[4-[4- (hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]-α,α-dimethylphenylacetic acid (Fexofenadine). In practice it is usually used as hydrochloride salt. |
| Therapeutic Function | Antihistaminic |
| General Description | Fexofenadine hydrochloride, (±)-4-[1-hydroxy-4-[4-(hydroxyldiphenylmethyl)- 1-piperinyl]butyl-α,α-dimethylbenzeneacetic acid (Allegra), occurs as a white to off-white crystalline powder that is freely soluble in methanol and ethanol, slightly soluble in chloroform and water, and insoluble in hexane. This compound is marketed as a racemate and exists as a zwitterion in aqueous media at physiological pH. Fexofenadine is a primary oxidative metabolite of terfenadine. Terfenadine was developed during a search for new butyrophenone antipsychotic drugs as evidenced by the presence of the N-phenylbutanol substituent. It also contains a diphenylmethyl-piperidine moiety analogous to that found in the piperazine antihistamines. |
| Biological Activity | Fexofenadine hydrochloride is a selective histamine H1 receptor antagonist (pKi = 8.1). Active metabolite of Terfenadine that displays non-sedating antiallergic effects. Fexofenadine hydrochloride counteracts histamine mediated immune suppression and improves immunotherapy response. |
| Biochem/physiol Actions | Fexofenadine is a non-sedating H1 histamine receptor antagonist. |
| Clinical Use | Antihistamine: Symptomatic relief of rhinitis and urticaria |
| Drug interactions | Potentially hazardous interactions with other drugs Antibacterials: effects possibly reduced by rifampicin. Antivirals: concentration possibly increased by ritonavir. Aluminium/magnesium containing antacids: reduced absorption – avoid for 2 hours. |
| Metabolism | Fexofenadine undergoes negligible metabolism (hepatic or non-hepatic); about 5% of the total dose is metabolised, mostly by the intestinal mucosa, with 0.5-1.5% of the dose undergoing hepatic biotransformation by the cytochrome P450 system. The major route of elimination is believed to be via biliary excretion while up to 10% of ingested dose is excreted unchanged through the urine. |
| Fexofenadine hydrochloride Preparation Products And Raw materials |