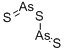| Chemical Properties | Arsenic trisulfide is a noncombustible, odorless, yellow or orange powder or red needles (changes to a different “red” form @ 170 C). |
| Chemical Properties | yellow to orange powder |
| Physical properties | Yellow or orange monoclinic crystal or powder; a red allotrope modification also known; density 3.46 g/cm3; melts at 310°C; boils at 707°C; insoluble in water; soluble in liquid ammonia and alkalies. |
| Occurrence | Arsenic sesquisulfide occurs in nature as the mineral orpiment. It is used as a pigment; in the manufacture of infrared-transmitting glass; in semiconductors and photoconductors; in pyrotechnics; in linoleum and oil cloth; for the removal of hairs from hides; and as a reducing agent. |
| Uses | Arsenic(III) sulfide is used in the treatment of acute promyelocytic leukemia. It is an inorganic photoresist used in the fabrication of photonic crystals and 3-D nanostructures. It is also used as an acousto-optic material, pigment, tanning agent and chalcogenide glass for infrared optics. Further, it is used as an analytical test for presence of dissimilatory arsenic-reducing bacteria (DARB). |
| Uses | manufacture of glass, particularly infrared-transmitting glass; manufacture of oil cloth, linoleum; in electrical semiconductors, photoconductors; as pigment; for depilating hides; in pyrotechnics. |
| Preparation | Arsenic sesquioxide may be prepared by heating arsenic trioxide with hydrogen sulfide:
As2O3 + 3 H2S → As2S3 + 3 H2O
Alternatively, it may be precipitated out from a solution of arsenous acid or arsenic trioxide in dilute hydrochloric acid by passing hydrogen sulfide into the solution:
2H3AsO3 + 3H2S → As2S3 + 6H2O. |
| General Description | A yellow or red crystalline solid or powder. Combustible. Insoluble in water. Toxic by inhalation (dust) and ingestion. |
| Air & Water Reactions | Insoluble in water. |
| Reactivity Profile | ARSENIC (III) SULFIDE is dissolved by alkalis and by hydrochloric acid (slowly). Decomposed by nitric acid. May react with oxidizing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. The reactions may be violent. ARSENIC (III) SULFIDE reacts with concentrated solutions of chloric acid with incandescence [Mellor Supp. II Part I:584 1956]. Generates toxic gaseous hydrogen sulfide on contact with acids. |
| Health Hazard | (Acute and sub-acute poisoning are not common.) Repeated inhalation causes irritation of nose, laryngitis, mild bronchitis. Ingestion causes weakness, loss of appetite, gastrointestinal disturbances, peripheral neuritis, occasional hepatitis. Contact with eyes causes irritation. Irritates skin, especially where moist; if not treated, may cause ulceration. |
| Fire Hazard | Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. |
| Industrial uses | As2S3 (orpiment) and As2S2 (realgar) have been used as early as 2000 BC as drugs, for example, to cure cancerous tumours, ulcers and other diseases of the time. Later, Galen (130 200 AD) recommended the application of a paste of arsenic sulfide against ulcers. |
| Safety Profile | Confirmed human carcinogen with experimental tumorigenic data. A poison. Reacts violently with H202, (m03 + S). When heated to decomposition or on contact with acid or acid fumes it emits highly toxic fumes of S02, H2S, and As. Reacts with water or steam to emit toxic and flammable vapors. |
| Potential Exposure | Arsenic trisulfide is used in the manufacture of glass, oil cloth, linoleum, electrical semiconductors, fireworks and used as a pigment. |
| Shipping | UN1557 Arsenic compounds, solid, n.o.s. inorganic, including arsenates, n.o.s.; arsenites, n.o.s.; arsenic sulfides, n.o.s.; and organic compounds of arsenic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials. |
| Incompatibilities | A strong reducing agent; avoid contact with oxidizers (such as perchlorates, peroxides, permanganates, chlorates, and nitrates) and potassium nitrate mixed with sulfur, since violent reactions occur. Water contact forms hydrogen sulfide. Incompatible with acids, halogens. Contact with acids or acid mists releases deadly arsine gas. |
| Waste Disposal | Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. |