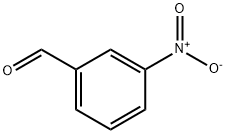
| Description | 3-Nitrobenzaldehyde, meta-nitrobenzaldehyde or m-nitrobenzaldehyde is an organic aromatic compound containing a nitro group meta-substituted to an aldehyde. 3-Nitrobenzaldehyde is the primary product obtained via the mono-nitration of benzaldehyde with nitric acid. |
| Chemical Properties | yellowish to yellow-brown granular powder |
| Uses | 3-Nitrobenzaldehyde is a benzaldhyde with a nitro group in the meta position. |
| Uses | 3-Nitrobenzaldehyde is used in the synthesis of other organic compounds, ranging from pharmaceuticals to plastic additives and intermediate for the processing of perfume and flavoring compounds and in the preparation of certain aniline dyes. |
| Preparation | 89 mL (1.7 mol) concentrated H2SO4 are filled in a 500 mL three-neck flask equipped with an internal thermometer and an addition funnel with pressure balance. Whilst cooling with an ice bath 45 mL (0.95 mol) fuming HNO3 are added carefully under stirring, the temperature must not exceed 10 °C. To this nitrating acid, 10.6 g (10.2 mL, 100 mmol) benzaldehyde are added under further cooling so that the temperature can be constantly kept at 15 °C (about 1 hour). The ice bath is removed and the reaction mixture is stored over night at room temperature.
The reaction mixture is poured in a 1 L beaker on 500 g crunched ice, the yellow precipitation is sucked off at 16 hPa over a Buechner funnel and washed with 200 mL of cold water. Crude yield (humid): 14.4 g
The humid crude product is dissolved in 125 mL tert-butyl methyl ether and then shaken out with 125 mL of a 5% NaHCO3 solution. The organic phase is dried over sodium sulfate, filtered and the solvent evaporated at a rotary evaporator. The residue is recrystallized from toluene / petroleumether (60-80 °C) by dissolving it in toluene whilst heating and then adding the double amount of petroleum ether in portions under ice cooling. The crystalllized light yellow 3-nitrobenzaldehyde is sucked off over a Buechner funnel. The product is dried over silica gel in an evacuated desiccator.
Yield: 8.0 g (53 mmol, 53%); mp 56 °C |
| Synthesis Reference(s) | Organic Syntheses, Coll. Vol. 3, p. 644, 1955
Tetrahedron Letters, 28, p. 1195, 1987 DOI: 10.1016/S0040-4039(00)95324-3 |
| Purification Methods | Crystallise the aldehyde from water or EtOH/water, then sublime it twice at 2mm pressure at a temperature slightly above its melting point. [Beilstein 7 IV 591.] |