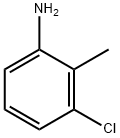
| Synthesis | It is obtained by chlorination and reduction of o-nitrotoluene.
1. Chlorination reaction
In o-nitrotoluene, add 2% ferric chloride as a catalyst, pass chlorine gas at 50-60 ° C until the relative density of the reaction solution reaches 1.27-1.29 (50 ° C), wash with 5% hydrochloric acid, and then Wash with water, and finally adjust pH to 7 with liquid caustic soda. Divide the water layer, evaporate the water, carry out vacuum distillation, collect 120-150 ℃ (5.33-8.0kPa) fractions, crystallize by cooling and filter to obtain 2-chloro-6-nitrotoluene.
2. Reduction reaction
Add hydrochloric acid and iron powder to the reaction pot, stir and heat to 90°C, slowly add melted 2-chloro-6-nitrotoluene, complete the addition, and reflux for 3 hours. Cooling, adding liquid alkali to adjust pH to 8, steam distillation to obtain 3-chloro-o-toluidine. |
| Chemical Properties | The chloromethylanilines are colorless or white crystalline solids or liquids, some have a mild fishy odor. |
| Chemical Properties | clear yellow to red or red-brown liquid |
| Uses | 3-Chloro-2-Methylaniline can be used for dyeing cotton, viscose, silk, nylon and acetate, as well as printing on cotton. It is mainly used for dyeing red, such as coupling with chromophenol AS, AS-BO, AS-ITR, etc. |
| Uses | Intermediate. |
| Preparation | By 1-Methyl-2-nitrobenzene chlorination byproducts 3-Chloro-2-methylbenzenamine?reduction. |
| Hazard | Toxic by ingestion or inhalation. |
| Safety Profile | Poison by ingestion. Mutation data reported. When heated to decomposition it emits toxic fumes of Cl and NOx. See also other chloro toluidine entries. |
| Potential Exposure | Most of the isomers are used in dyestuff manufacture. The 3-chloro-para isomer is used to kill birds. It is marketed as pelleted bait for control of bird populations. |
| Incompatibilities | Incompatible with oxidizers, strong acids; chloroformates, and acid anhydrides, isocyanates, aldehydes forming fire and explosive hazards. |