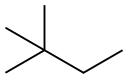
| Chemical Properties | 2,2-dimethylbutane is the chain isomeric form of hexane. It is a colorless and flammable liquid with a specific gravity of 0.6444. Insoluble in water, miscible with alcohol, ether, acetone, benzene and petroleum ether. The solubility is similar to that of hexane and 2-methylpentane. |
| Physical properties | Clear, colorless liquid with a mild petroleum-like odor. Liquid quickly evaporates and forms conbustible fumes. An odor threshold concentration of 20 ppmv was reported by Nagata and Takeuchi (1990). |
| Uses | 2,2-Dimethylbutane is an acyclic alkane. Its Research Octane Number (RON) has been reported to be 91.8. It is used to Component of high-octane motor and aviation fuels, intermediate for agricultural chemicals. |
| Preparation | 2,2-Dimethylbutane can be synthesised by the hydroisomerisation of 2,3-dimethylbutane using an acid catalyst.
By the thermal or catalytic union (alkylation) of ethylene and isobutane, both recovered from refinery gases. |
| Production Methods | 2,2-Dimethylbutane is produced from crude oil, natural liquid gases, and petroleum refining processes. |
| Application | 2,2-Dimethylbutane may be employed as probe to investigate the nature of possible active sites in supported metal catalysts.
Some of the reported applications of 2,2-dimethylbutane include:
Palladium-catalyzed radical oxidative alkoxycarbonylation to prepare various alkyl carboxylates.
Iridium-iron-catalyzed tandem dehydrogenation-isomerization-hydrosilylation to synthesize corresponding silane. |
| General Description | Colorless liquid with an odor of gasoline. Less dense than water and insoluble in water. Hence floats on water. Irritating vapor. Flash point -54°F. |
| Air & Water Reactions | Highly flammable. Insoluble in water. |
| Reactivity Profile | 2,2-Dimethylbutane may be incompatible with strong oxidizing agents like nitric acid. Charring may occur followed by ignition of unreacted material and other nearby combustibles. In other settings, mostly unreactive. Not affected by aqueous solutions of acids, alkalis, most oxidizing agents, and most reducing agents. When heated sufficiently or when ignited in the presence of air, oxygen or strong oxidizing agents, burns exothermically to produce carbon dioxide and water. |
| Hazard | Highly flammable, dangerous fire and explosion risk, explosion limits 1.2–7% |
| Health Hazard | Inhalation causes dizziness, nausea, and vomiting; concentrated vapor may cause unconsciousness and collapse. Contact with liquid causes irritation of eyes; repeated contact may produce irritation of skin. Ingestion causes irritation of stomach. Aspiration causes severe lung irritation, rapidly developing pulmonary edema, and central nervous system excitement followed by depression. |
| Safety Profile | Probably an irritant and narcotic in high concentration. A very dangerous fire and explosion hazard when exposed to heat or flame; can react vigorously with oxidzing materials. Keep away from heat or open flame. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. |
| Environmental fate | Photolytic. Reported photooxidation rate constants for the reaction of 2,2-dimethylbutane with OH radicals at 297, 299, and 300 K are 2.59 x 10-12, 6.16 x 10-12, and 2.59 x 10-12 cm3/molecule?sec, respectively (Atkinson, 1985, 1990).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water vapor. 2,2- Dimethylbutane will not hydrolyze because it has no hydrolyzable functional group. |
| Purification Methods | Distil it azeotropically with MeOH, then wash it with water, dry (Na2SO4) it, and distil it. [Beilstein 1 IV 367.] |