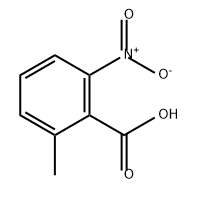
| Chemical Properties | light orange to yellow-brown crystalline powder |
| Uses | 2-Methyl-nitrobenzoic acid acts as herbicide due to the anthranilic acid moiety within the substructure. |
| General Description | Pale yellow needles or tan powder. |
| Air & Water Reactions | Insoluble in water. |
| Reactivity Profile | 2-METHYL-6-NITROBENZOIC ACID is a nitrated carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called “neutralizations”, are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. |
| Fire Hazard | Flash point data for 2-METHYL-6-NITROBENZOIC ACID are not available; however, 2-METHYL-6-NITROBENZOIC ACID is probably combustible. |