| Chemical Properties | 5-Nitro-o-toluidine is a yellow, crystalline solid. |
| Chemical Properties | yellow powder |
| Uses | 2-Methyl-5-nitroaniline may be employed as analytical standard for the surfactant mediated transformations (?254nm) of 2,4-dinitrotoluene. |
| Uses | 5-Nitro-o-toluidine is used in the synthesis of numerous dyes. |
| Preparation | 2-Methyl-5-nitrobenzenamine?in concentrated sulfuric acid nitration. |
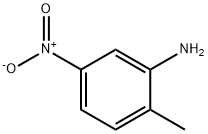
| Definition | ChEBI: A C-nitro compound in which the nitro compound is meta to the amino group and para to the methyl group of o-toluidine. |
| General Description | Bright yellow powder. |
| Air & Water Reactions | Insoluble in water. 2-Methyl-5-nitroaniline is sensitive to moisture, light, or prolonged exposure to air. |
| Reactivity Profile | 2-Methyl-5-nitroaniline is incompatible with strong oxidizing agents, acids, acid chlorides, acid anhydrides and chloroformates. |
| Fire Hazard | Flash point data for 2-Methyl-5-nitroaniline are not available; however, 2-Methyl-5-nitroaniline is probably combustible. |
| Safety Profile | Confirmed carcinogen with experimental carcinogenic data. Moderately toxic by ingestion. Mutation data reported. Decomposes exothermically when heated to 150℃. When heated to decomposition it emits toxic fumes of NOx. See also NITRO COMPOUNDS OF AROMATIC HYDROCARBONS. |
| Carcinogenicity | There are no data available for evaluating carcinogenic risk to humans. When 5-nitro-o-toluidine was administered in a dietary feeding study to F344 rats (50 or 100 ppm) and B6C3F1 mice (1200 or 2300 ppm) of both sexes, hepatocellular carcinomas were produced in mice but not in rats. |
| Shipping | UN2660 Nitrotoluidines (mono), Hazard Class: 6.1; Labels: 6.1-Poisonous materials. |
| Purification Methods | Acetylate the aniline, and the acetyl derivative is crystallised to constant melting point; then hydrolyse it with 70% H2SO4 and the free base is regenerated by treatment with NH3 [Bevan et al. J Chem Soc 4284 1956]. [Beilstein 12 H 844. 12 IV 1807.] |
| Incompatibilities | Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acid chlorides; acid anhydrides; chloroformates. |
| Waste Disposal | Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. |