| (1S)-(+)-Camphor-10-sulphonic acid Basic information |
| Synthesis |
| Product Name: | (1S)-(+)-Camphor-10-sulphonic acid |
| Synonyms: | D-CAMPHORSULFONIC ACID;D(-)-CAMPHORSULPHONIC ACID;D-REYCHLER’S ACID;D-OXO-10-BORNANESULFONIC ACID;(+)-BETA-CAMPHORSULFONIC ACID;(+)-CAMPHOR-10-SULFONIC ACID;(+)-CAMPHOR-10-SULFONIC ACID (BETA);(1S)-(+)-CAMPHOR-10-SULFONIC ACID |
| CAS: | 3144-16-9 |
| MF: | C10H16O4S |
| MW: | 232.3 |
| EINECS: | 221-554-1 |
| Product Categories: | chiral;Pharmaceutical Intermediates;FINE Chemical & INTERMEDIATES;Chiral Compounds;Bicyclic Monoterpenes;Biochemistry;for Resolution of Bases;Optical Resolution;Reagents for Oligosaccharide Synthesis;Synthetic Organic Chemistry;Terpenes;Peptide;Sulfur & Selenium Compounds;3144-16-9;3597-91-9 |
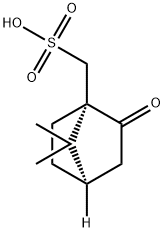
| Mol File: | 3144-16-9.mol |
 |
| (1S)-(+)-Camphor-10-sulphonic acid Chemical Properties |
| Melting point | 196-200 °C (dec.)(lit.) |
| alpha | 22 º (589nm, c=20, H2O 25 ºC) |
| Boiling point | 344.46°C (rough estimate) |
| density | 1.2981 (rough estimate) |
| refractive index | 21.5 ° (C=5, H2O) |
| storage temp. | Store below +30°C. |
| solubility | Exhibit moderate solubility in HCCl. |
| pka | 1.17±0.50(Predicted) |
| form | Crystalline Powder |
| color | White to off-white |
| PH | 0.3 (200g/l, H2O) |
| optical activity | [α]20/D +21.5±1°, c = 10% in H2O |
| Water Solubility | SOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,1734 |
| BRN | 2809675 |
| Stability: | Stable. Protect from moisture. Incompatible with strong oxidizing agents, strong bases. |
| InChIKey | MIOPJNTWMNEORI-GMSGAONNSA-N |
| CAS DataBase Reference | 3144-16-9(CAS DataBase Reference) |
| EPA Substance Registry System | Bicyclo[2.2.1]heptane-1-methanesulfonic acid, 7,7-dimethyl-2-oxo-, (1S,4R)- (3144-16-9) |
| Safety Information |
| Hazard Codes | C |
| Risk Statements | 34 |
| Safety Statements | 26-36/37/39-45-25-27 |
| RIDADR | UN 3261 8/PG 2 |
| WGK Germany | 3 |
| RTECS | ED1550000 |
| F | 3-10 |
| TSCA | Yes |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 29147090 |
| Provider | Language |
|---|---|
| 1S-(+)-Camphor-10-sulfonic acid | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| (1S)-(+)-Camphor-10-sulphonic acid Usage And Synthesis |
| Synthesis | (1S)-(+)-Camphor-10-sulphonic acid can be obtained from natural camphor by brominated sulfonation . |
| Chemical Properties | white to light beige powder |
| Uses | (1S)-(+)-Camphor-10-sulfonic acid is used as stabilizer. (+)-(1S)-camphor-10-sulfonic acid being a very effective resolving agent. . Chiral polyaniline(PANI) nanofibers were synthesized via facilely potentiostatic electropolymerization method without template in the presence of(1S)-(+)camphor-10-sulfonic acid(D-CSA) or(1R)-(-)camphor-10-sulfonic acid(L-CSA) as the dopant. Synthesis of QUATs derived from (1S)-(+)camphor-10-sulfonic acid. |
| Uses | Used as a resolving agent, and as a catalyst for coupling dipeptides. |
| Definition | ChEBI: (S)-camphorsulfonic acid is the S enantiomer of camphorsulfonic acid. It is a conjugate acid of a (S)-camphorsulfonate. It is an enantiomer of a (R)-camphorsulfonic acid. |
| Purification Methods | Crystallise the acid from ethyl acetate and dry it under vacuum (deliquescent). [Loudon J Chem Soc 823 1933, Komppa J Prakt Chem 162 19 1943, Beilstein 11 IV 642.] See above for RS-isomer. |
| (1S)-(+)-Camphor-10-sulphonic acid Preparation Products And Raw materials |