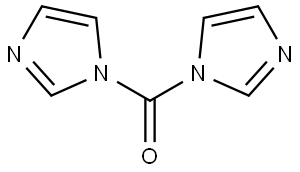
| Chemical Properties | The substance appears as a white to slightly pale-yellow crystalline powder with a melting point range of 115.5-116°C. It exhibits poor solubility in water, while displaying solubility in alcohol and ether. |
| Uses | CDI, also known as 1,1′-carbonyldiimidazole, is a commonly used peptide coupling reagent in the synthesis of peptides and nucleoside triphosphates. It usually contains approximately 10% imidazole and reacts rapidly with carboxylic acids to produce acyl imidazoles. Later, it readily reacts with amines to create amides. In addition, CDI is useful in converting alcohols and amines into a range of chemical compounds, including carbamates, esters, and ureas. |
| Application | Peptide coupling reagent1,1′-Carbonyldiimidazole acts as a coupling reagent and utilized for coupling of amino acids in order to prepare peptide in organic synthesis. It is also used in the preparation of beta-keto sulfones, sulfoxides and beta-enamino acid derivatives. It is used to convert alcohols and amines into carbamates, esters, and ureas. It is involved in the preparation of formylized imidazole by reaction with formic acid. Further, it is used in the synthesis of dipolar polyamides compounds. In addition to this, it is considered as an equivalent of phosgene and used to prepare asymmetric bis alkyl carbonate. |
| Preparation | 1,1′-Carbonyldiimidazole has been prepared by the reaction of imidazole and phosgene in anhydrous benzene and anhydrous tetrahydrofuran. It has also been obtained by the reaction of 1-(trimethylsilyl)imidazole and phosgene in anhydrous benzene, but that method offers no advantages that justify the more extensive preparative effort required.
DOI: 10.15227/orgsyn.048.0044 |
| General Description | 1,1′-Carbonyldiimidazole (N,N′-carbonyldiimidazole) is a versatile peptide-forming reagent. |
| Purification Methods | Crystallise it from *benzene or tetrahydrofuran in a dry-box and store it dry. [Hearn Methods Enzymol 135 102 1987, Beilstein 23/4 V 245.] |
| Precautions | 1,1′-Carbonyldiimidazole is incompatible with oxidizing agents and strong acids and should not be stored or handled near them. Store in a refrigerator, under inert gas and protected from moisture. This substance is heat sensitive and moisture sensitive. |