| Physical Properties | White crystalline solid; orthorhombic structure; refractive index 1.818; Mohs hardness 4.3; decomposes at 300°C forming zinc oxide; practically insoluble in water, 10 mg/L at 15°C; soluble in acids, alkalis, and ammonium salt solutions. |
| Occurrence and Uses | Zinc carbonate occurs in nature as mineral smithsonite and zincspar. The compound is used in ceramics and fire proofing filler for rubber and plastics.Also, it is used in lotions, ointments, cosmetics, and as a topical antiseptic. |
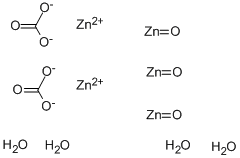
| Production | Zinc carbonate is derived from its mineral smithsonite. Also, the compound may be prepared by the reaction of sodium bicarbonate with a soluble zinc salt:
ZnCl2 + NaHCO3 → ZnCO3 + NaCl + HCl |
| Chemical Properties | White, crystalline powder. Evolves carbon dioxide at 300C. Soluble in acids, alkalies, and ammonium salt solutions; insoluble in water. |
| Chemical Properties | Smithsonite is zinc carbonate, ZnCO3, a hexagonal mineral with a rhombohedral cleavage. It is a brittle mineral; hardness, 4–4.5; specific gravity, 4.3–4.5; luster, vitreous to dull; color, usually white, but may be colored yellowish or brownish or perhaps blue or green due to impurities. It is translucent to opaque. |
| Uses | Antiseptic (topical); astringent. |
| Uses | Zinc Carbonate is used in method for producing Zinc Carbonate using electric furnace dust. |
| Definition | ChEBI: Zinc carbonate is an organooxygen compound. |
| General Description | A white crystalline solid or powder that is insoluble in water. The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. Used in pharmaceuticals, to make other zinc compounds, as a feed additive. |
| Reactivity Profile | Zinc carbonate reacts with acids to generate carbon dioxide and a zinc-containing solution. Dissolves in bases and in solutions of ammonium salts. |
| Health Hazard | INHALATION OF DUST OR FUMES: Dry throat, cough and chest discomfort. Fever and sweating. SKIN: Astringent. INGESTION: May cause nausea and vomiting. |
| Fire Hazard | Behavior in Fire: Could decompose to liberate CO 2 |
| Flammability and Explosibility | Notclassified |
| Safety Profile | An experimental teratogen. When heated to decomposition it emits toxic fumes of CO and Zn. |