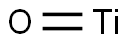| Chemical Properties | bronze pellets; fcc; weakly basic oxide with no important industrial uses [HAW93] [STR93] [KIR83] |
| Chemical Properties | Titanium monoxide, TiO, has a rock-salt structure but can exist with both oxygen and titanium vacancies. For stoichiometric TiO, the lattice parameter is 417 pm, but varies from ca 418 pm at 46 atom % to 4162 pm at 54 atom % oxygen. Apparently, stoichiometric TiO has ca 15% of the Ti and O sites vacant. At high temperatures (>900 °C (1,652 °F)), these vacancies are randomly distributed; at low temperatures, they become ordered. Titanium monoxide may be made by heating a stoichiometric mixture of titanium metal and titanium dioxide powders at 1,600 °C (2,912 °F). |
| Uses | Used for vacuum deposition. Also applications include sunscreens and UV-blocking in cosmetics, varnishes, wood preservatives, textile fibers, and packaging films. Also used as paint pigment, opacifying agent, welding rod fluxes, optical coatings, as a catalyst, ceramic finish coat, plastics, elastomers, coated fabrics, printing inks, roofing granules, glass, and in glazes. Also identified as Titanium monoxide. |
| Definition | ChEBI: Titanium(II) oxide is a titanium oxide containing approximately equal numbers of titanium and oxygen ions. |