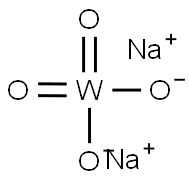| Chemical Properties | Sodium tungstate is a sodium salt of orthotungstate. It is a colorless crystals or white crystalline powder. Soluble in water; insoluble in alcohol and acids. noncombustible. Sodium tungstate has three crystalline forms, δ, γ and β. The transition temperature from δ to γ is about 570℃, and from γ to β is about 585℃. In hydrogen is heated to 700 ℃ also does not change, to 1100 ℃ will become metal tungsten. |
| Uses | Intermediate in preparation of tungsten compounds, (e.g., phosphotungstate), reagent, fire-proofing fabrics and cellulose, alkaloid precipitant. |
| Definition | ChEBI: Sodium tungstate is an inorganic sodium salt having tungstate as the counterion. Combines with hydrogen peroxide for the oxidation of secondary amines to nitrones. It has a role as a reagent. It contains a tungstate. |
| Preparation | Sodium tungstate is obtained by digestion of tungsten ores, the economically important representatives of which are tungstates, in base. Illustrative is the extraction of sodium tungstate from wolframite:
Fe/MnWO4 + 2 NaOH + 2 H2O → Na2WO4?2H2O + Fe/Mn(OH)2
Sodium tungstate can also be produced by treating tungsten carbide with a mixture of sodium nitrate and sodium hydroxide in a fusion process which overcomes the high exothermicity of the reaction involved. |
| Flammability and Explosibility | Notclassified |
| Safety Profile | Poison by ingestion, intravenous, intramuscular, and subcutaneous routes. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of Na2O. See also TUNGSTEN COMPOUNDS. |