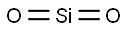| General Description | Silicon dioxide occurs almost everywhere on earth. It is one of the most important and abundant oxides on earth, constituting about 60% weight of the earth’s crust as silica itself or in combination with other metal oxides in silicates. It commonly is found as sand in the vast ocean and river shores, their beds, deserts, rocks, and minerals.
Silicon dioxide exists in several structural forms: polymorphic crystalline silica, synthetic quartz crystals, amorphous silica, and vitreous silica. This classification is not complete as there are other forms of silica synthesized for specialized applications. |
| Uses | IndustryApplicationRole/benefitFoodPowdered foods, such as salt, many spices, etc.Ant-caking agent/ when added to a mixture, prevents its ingredients from binding togetherNutritional health food supplementsSource of silicon/maintain healthy strong bones and joints and minimizes aluminum effects on the bodyWine, beer and juiceFining agentChemical manufactureManufacture of silicon compounds and sodium silicateRaw materialConstructionProduction of portland cementRaw materialSand castingMain ingredient/high melting pointGlassHigh purity silica glassRaw material/high temperature and corrosion resistanceDomestic glass and optical devicesEssential componentCeramicsManufacture of ceramic glazeMain constituents/forms glass when heated to bind others ingredients togetherMetallurgyManufacture of silicon alloysRaw material or additivePharmaceuticalDrug tablets makingFlow agent/aids powder flow when tablets are formedPreventing Alzheimer’s diseaseEffective components/minimizes aluminum effects on the body which may cause Alzheimer’s diseaseElectronicsFiber optic cablesRaw material/high level of heat conductivity and low rate of transmission lossWire insulationRaw material/high melting point and good insulating propertySemi-conductorsSource of siliconPiezoelectric transducerMain component/can convert mechanical energy to electrical energy and vice-versaOthersRefractory materialsMain component/high melting point and high shock resistanceRubber and plasticsAdditive/improves wearing capacityDNA ExtractionSource of silicon/has binding properties which help to isolate the strands of DNAManufacture of silica gelSource of silicon/hygroscopic propertyDefoamingDefoamer componentSilica-based aerogelSource of siliconHydraulic fracturingThickening agent |
| Chemical Properties | white crystals or powder |
| Chemical Properties | Diatomaceous earth is a transparent to gray, odorless amorphous powder. |
| Chemical Properties | Amorphous silica, the noncrystalline form of SiO2, is a transparent to gray, odorless, amorphous powder |
| Uses | Silica (SiO2) (RI: 1.48) is mined from deposits of diatomaceous soft chalk-like rock (keiselghur). This is an important group of extender pigments, which is used in a variety of particle sizes. They are used as a flatting agent to reduce gloss of clear coatings and to impart shear thinning flow properties to coatings. They are relatively expensive. |
| Uses | silica is also known as silicone dioxide. Silica has a variety of applications: to control a product’s viscosity, add bulk, and reduce a formulation’s transparency. It can also function as an abrasive. In addition, it can act as a carrier for emollients, and may be used to improve a formulation’s skin feel. Spherical silica is porous and highly absorbent, with absorption capabilities roughly 1.5 times its weight. A typical claim associated with silica is oil control. It is found in sunscreens, scrubs, and wide range of other skin care, makeup, and hair care preparations. It has been successfully used in hypoallergenic and allergy-tested formulations. |
| Uses | Functionalized RAFT agent for controlled radical polymerization; especially suited for the polymerization of styrene; acrylate and acrylamide monomers. Azide group can be used to conjugate to a variety of alkyne-functionalized biomolecules. Chain Transfer Agent (CTA). |
| Uses | SDS mixture of sodium alkyl sulfates consisting chiefly of sodium lauryl sulfate |
| Uses | manufacture of glass, water glass, refractories, abrasives, ceramics, enamels; decolorizing and purifying oils, petroleum products, etc.; in scouring- and grinding-compounds, ferrosilicon, molds for castings; as anticaking and defoaming agent. |
| Uses | Silicon(IV) oxide, amorphous is used as carriers, processing aids, anti-caking and free-flow agents in animal feed. Defoamer applications such as paint, food, paper, textile and other industrial applications. Synthetic silicon dioxides are used as a rheology control agent in plastics. It is also used to manufacture adhesives, sealants and silicones. |
| Definition | ChEBI: A silicon oxide made up of linear triatomic molecules in which a silicon atom is covalently bonded to two oxygens. |
| General Description | contains sodium stabilizing counterion |
| Hazard | Not toxic if ingested, inhaled silica dust can cause silicosis; carcinogen. |
| Agricultural Uses | Silica is silicon dioxide, one of the most abundant materials on the earth’s crust. Quartz is an example of silica. It is used as a filler in fertilizers, and also, in the manufacture of glass, ceramics, abrasives, rubber and cosmetics. |
| Safety Profile | The pure unaltered form is considered a nuisance dust. Some deposits contain small amounts of crystahne quartz and are therefore fibrogenic. When diatomaceous earth is calcined (with or without fluxing agents) some sdica is converted to cristobalite and is therefore fibrogenic. Tridymite has never been detected in calcined batomaceous earth. See also other silica entries |
| Potential Exposure | Diatomaceous earth is used as a filtering agent and as a filler in construction materials, pesticides, paints, and varnishes. The calcined version (which has been heat treated) is the most dangerous and contains crystallized silica, and should be handled as silica. See also other entries on silica |
| Shipping | This material is not singled out by DOT in its Performance-Oriented Packaging Standards. |
| Purification Methods | Purification of silica for high technology applications uses isopiestic vapour distillation from concentrated volatile acids and is absorbed in high purity water. The impurities remain behind. Preliminary cleaning to remove surface contaminants uses dip etching in HF or a mixture of HCl, H2O2 and deionised water [Phelan & Powell Analyst 109 1299 1984]. |
| Incompatibilities | Silica, amorphous is a noncombustible solid. Generally unreactive chemically. Incompatible with fluorine, oxygen difluoride, chlorine trifluoride. Soluble in molten alkalis and reacts with most metallic oxides at high temperature. |
| Waste Disposal | Sanitary landfill. |