| Potassium hexafluorophosphate Basic information |
| Product Name: | Potassium hexafluorophosphate |
| Synonyms: | Potassiumhexafluorophosphate,99.5%;Potassium fluorophosphate;Potassium hexafluorophosphate, extra pure, 99%;Kaliumhexafluorophosphat;Phosphate,hexafluoro-,potassium;POTASSIUM HEXAFLUOROPHOSPHATE(V): 99.5%;POTASSIUM HEXAFLUOROPHOSHATE;Potassium hexafluorophosphate, 99% min |
| CAS: | 17084-13-8 |
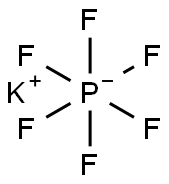
| MF: | F6KP |
| MW: | 184.06 |
| EINECS: | 241-143-0 |
| Product Categories: | Preservative;metal fluorophosphate complex;Inorganic Salts;Potassium Salts;PotassiumMetal and Ceramic Science;Salts;Synthetic Reagents;Potassium;bc0001;17084-13-8 |
| Mol File: | 17084-13-8.mol |
 |
| Potassium hexafluorophosphate Chemical Properties |
| Melting point | 575 °C (lit.) |
| Boiling point | decomposes [STR93] |
| density | 2.75 g/mL at 25 °C (lit.) |
| storage temp. | Refrigerator |
| solubility | Methanol (Slightly), Water (Soluble) |
| form | Powder |
| Specific Gravity | 2.55 |
| color | White |
| Water Solubility | 93 g/L (25 ºC) |
| Sensitive | Hygroscopic |
| Stability: | Stable. Incompatible with strong acids. Thermal decomposition may generate HF, phosphorus oxides and phosphine. |
| InChIKey | YZDGRYDIGCWVND-UHFFFAOYSA-N |
| CAS DataBase Reference | 17084-13-8(CAS DataBase Reference) |
| EPA Substance Registry System | Phosphate(1-), hexafluoro-, potassium (17084-13-8) |
| Safety Information |
| Hazard Codes | C,Xi |
| Risk Statements | 34-20/21/22 |
| Safety Statements | 26-27-36/37/39-45 |
| RIDADR | UN 3260 8/PG 2 |
| WGK Germany | 1 |
| Hazard Note | Irritant |
| TSCA | T |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 28269090 |
| Provider | Language |
|---|---|
| Potassium hexafluorophosphate | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Potassium hexafluorophosphate Usage And Synthesis |
| Chemical Properties | white crystalline powder |
| Uses | A soluble neutral salt, Potassium hexafluorophosphate can be used for synthesis. |
| Uses | Potassium Hexafluorophosphate is used in preparation of Moxifloxacin derivatives and its application. |
| Hazard | Toxic by ingestion. |
| Purification Methods | Crystallise it from alkaline aqueous solution, using polyethylene vessels, or from 95% EtOH, and dry it in a vacuum desiccator over KOH. [Kloditz Z Anorg Allgem Chem 284 144 1956, Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 196 1963.] |
| Potassium hexafluorophosphate Preparation Products And Raw materials |