| Phosphorus pentoxide Basic information |
| description Chemical Properties Hazardous characteristics Reference quality standards Reaction with water Toxicity Water absorption Uses Production method |
| Product Name: | Phosphorus pentoxide |
| Synonyms: | Phosphorus pentoxide, 99.99+% metals basis;PhosphorusPentoxide99.999%;PhosphorousPentoxideExtraPure;PhosphorousPentoxideP2O5;PhosphorusPentoxide99.99%;PhosphorousPentoxideGr;PhosphorousPentoxide98%;phosphorusoxide[p2o5] |
| CAS: | 1314-56-3 |
| MF: | O5P2 |
| MW: | 141.94 |
| EINECS: | 215-236-1 |
| Product Categories: | element oxide;Inorganics;Organics;Phosphate;Inorganic Chemicals;1314-56-3 |
| Mol File: | 1314-56-3.mol |
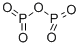 |
| Phosphorus pentoxide Chemical Properties |
| Melting point | 340 °C (lit.) |
| Boiling point | 122 °C (1 mmHg) |
| density | 2.3 g/mL at 25 °C (lit.) |
| vapor density | 4.9 (vs air) |
| vapor pressure | 1 mm Hg ( 384 °C) |
| refractive index | 1.433-1.436 |
| Fp | 340-360°C |
| storage temp. | no restrictions. |
| solubility | Soluble in sulfuric acid. Insoluble in acetone and ammonia. |
| form | Very Deliquescing Powder |
| color | White |
| Specific Gravity | 2.39 |
| Odor | Pungent odour |
| PH Range | <2 |
| PH | 1 (5g/l, H2O, 20℃) |
| Water Solubility | Soluble in sulfuric acid. Insoluble in acetone and ammonia. Decomposes in water. |
| Sensitive | Moisture Sensitive |
| Merck | 14,7355 |
| Sublimation | 340-360 ºC |
| Stability: | Stability Stable, but reacts violently with water, alcohols, metals, sodium, potassium, ammonia, oxidizing agents, HF, peroxides, magnesium, strong bases. |
| InChIKey | YWEUIGNSBFLMFL-UHFFFAOYSA-N |
| CAS DataBase Reference | 1314-56-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Diphosphorus pentoxide(1314-56-3) |
| EPA Substance Registry System | Phosphorus pentoxide (1314-56-3) |
| Safety Information |
| Hazard Codes | C |
| Risk Statements | 35 |
| Safety Statements | 22-26-45 |
| RIDADR | UN 1807 8/PG 2 |
| WGK Germany | 1 |
| RTECS | TH3945000 |
| F | 3-10 |
| TSCA | Yes |
| HS Code | 2809 10 00 |
| HazardClass | 8 |
| PackingGroup | II |
| Hazardous Substances Data | 1314-56-3(Hazardous Substances Data) |
| Provider | Language |
|---|---|
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
| Phosphorus pentoxide Usage And Synthesis |
| description | phosphorus pentoxide, also known as phosphoric anhydride, is phosphorus oxides generated by combustion of white phosphorus, yellow or red phosphorus in dry air, soft white powder or colorless monoclinic crystal at room temperature, easily deliquescence, melting point is 580~585 ℃, relative density is 2.39, sublimation is 347 ℃. When heated under pressure to 563 ℃, crystalline substance translates to amorphous glass (melt). It is insoluble in acetone, ammonia, soluble in sulfuric acid. phosphorus pentoxide is easy to absorb moisture from the air, and have a strong dehydration, can even dehydrate concentrated sulfuric acid to produce sulfur trioxide, is a potent desiccant, reacts violently with water and emits heat and white smoke. Per mole of phosphorus pentoxide releases 68 kilocalories, usually generate metaphosphate with cold water, and generate mainly phosphoric acid with hot water. According to the amount of water added, it can generate different pentavalent phosphorus oxyacid, such as metaphosphoric acid, pyrophosphoric acid, orthophosphoric acid, etc. phosphorus pentoxide is used to make high purity phosphoric acid, as well as gas and liquid desiccant, organic synthetic dehydrating agent. |
| Chemical Properties | Phosphorus pentoxide (P2O5) or phosphoric anhydride is a white, highly deliquescent solid which sublimes at 300°C and reacts violently with water to give orthophosphoric acid. It is prepared by burning elemental phosphorus in excess oxygen, and then purified by sublimation. The compound is used as a drying and dehydrating agent; for example, the amides are converted into nitriles, and sulphuric acid is converted to sulphur trioxide (SO3). The fertilizer phosphorus is specified as the weight percentage of phosphorus pentoxide. |
| Hazardous characteristics | Non-combustible. But encountering water and organic materials such as wood, cotton or straw, it reacts violently to release heat, may cause a fire. Contacting Water, it can emit a lot of smoke and heat. The case of the moisture, it corrodes most metals slightly. A strong local irritation, vapor with dust can severely irritate the eyes, mucous membranes, skin and respiratory system, and corrode the skin, mucous membranes. Even the dust concentration of 1mg/m3, it is unbearable. The above information is edited by the chemicalbook of Yan Yanyong. |
| Reference quality standards | Item Index Chemically pure analytically pure specific level Content (phosphorus pentoxide) ≥99% ≥99.5% ≥99.5% Active R value 1.2 1.7 2.0 Reducing substances (in terms P2O3) ≤0.02% ≤0.01% ≤0.005% Clarity test Pass Pass Pass Water insoluble matter ≤0.02% ≤0.01% ≤0.01% Total nitrogen (N) ≤0.002% ≤0.001% ≤0.001% Heavy Metals (in Pb) ≤0.002% ≤0.001% ≤0.001% Iron content (Fe) ≤0.002% ≤0.001% ≤0.001% Arsenic content (As) ≤0.01% ≤0.005% ≤0.0025% |
| Reaction with water | It reacts with cold water to produce extremely toxic metaphosphate: P2O5 + H2O = 2HPO3 and reacts with hot water to produce ontoxic orthophosphate: P2O5 + 3H2O = (heating) 2H3PO4, an exothermic reaction, the phenomenon is not very intense. |
| Toxicity | Phosphorus pentoxide smoke irritates mucous membranes, has irritation and burning effects for skin (tissue dehydration). Maximum allowable concentration is 1mg/rn3. Production equipment and pipelines should be closed, and maintain good ventilation. Production workers should wear labor protective equipments and wear protective respirator, when there are phosphorus pentoxide vapor and smoke , should wear protective masks. Pay attention to protect the skin, do not import, or meet the eye. If accidentally touches the skin, immediately wash with clean water. |
| Water absorption | There is a strong water imbibition, and easily deliquescent in the air, corrosive to the skin. The maximum allowable concentration in the workplace is 1mg/m3. The drying efficiency of Phosphorus pentoxide is 1 cubic meter 0.00001 grams of water vapor content at 298K, namely dried by phosphorus pentoxide, water left per cubic meter of air is up to 0.00001 gram, phosphorus pentoxide can make sulfuric acid, nitric acid dehydration. When the amount of phosphorus pentoxide and water substance is 1: 6, phosphorus pentoxide can convert to orthophosphoric acid. |
| Uses | Used as a drying agent, dehydrating agent, sugar refining agent, and used for the preparation of phosphoric acid, phosphorous compounds and aerosol, etc. Used as a semiconductor silicon doped source, dehydration drying agent, organic synthetic condensing agent and surfactant, it is also used for preparation of high purity phosphoric acid. It is used as raw material for the production of high purity phosphoric acid, phosphates and phosphate, also used for the preparation of phosphorus pentoxide sol and H-based aerosol. It can be used as a desiccant of gases and liquids, dehydrating agent for organic synthesis, antistatic agents of synthetic fiber and sugar refining agent. It is also used in the manufacture of optical glass, UV transparent glass, insulating glass, microcrystalline glass and opaque glass, etc, so as to improve the dispersion coefficient and UV light through ability of the glass. Also used in the production of pharmaceuticals, pesticides, surfactants. It is used as the semiconductor dopant, and raw material for the preparation of high purity phosphoric acid. It is used as desiccant and dehydrating agent, condensing agent in organic synthesis. High purity grade phosphorus pentoxide can be used as dopant for drawing optical fiber preform, N type dopant for the manufacture of integrated circuits, used for the synthesis of optical crystal. |
| Production method | Reagent grade phosphorus pentoxide is as raw material, purified by burning sublimation in sufficiently dry oxygen gas stream, trapping sublimate by the condenser, to obtain high-purity phosphorus pentoxide products. Yellow phosphorus is heated and melted by oxidation method, transfered to the oxidation furnace for oxidation combustion reaction with dry air (via dehydration of concentrated sulfuric acid), phosphorus pentoxide produced is sublimated by heat, cold in the top of burners and falls into interior settling chamber at the bottom, to prepare pentoxide products. Exhaust escapes from the oxidation furnace to a cyclone to recover phosphorus pentoxide dust. Recycling and the settling chamber finished product are together packaged as a finished product. P4 + 5O2 → 2P2O5 |
| Chemical Properties | Phosphorus pentoxide is a white crystalline solid. |
| Physical properties | White, deliquescent, powdery solid; exhibits polymorphism; converts to several different crystalline forms on heating; the commercial material consists of hexagonal crystals; the hexagonal crystals on very rapid heating first melt at 420°C and then resolidify immediately to glassy orthorhombic crystals; slow heating of hexagonal crystals causes melting at 340°C which, on solidification, gives the same metastable orthorhombic form; the glassy material melts at about 580°C to a colorless and heavily viscous liquid; sublimes at 360°C; density of the commercial product 2.39g/cm3; reacts with water. |
| Uses | It is used as a dehydrating agent, in organic synthesis, and in hydrocarbon analysis. |
| Uses | Phosphorus(V) oxide is used as a drying and dehydrating agent, a condensation reagent in organic synthesis and a laboratory reagent. It is also used in sugar refining and in fire extinguishing. Phosphorus pentoxide in DMSO forms an Onodera reagent which oxidizes alcohols. It is capable of converting mineral acids to anhydrides. |
| Uses | Drying and dehydrating agent. Condensing agent in organic synthesis. |
| Preparation | Phosphorus pentoxide is prepared by burning phosphorus in a plentiful supply of dry air or oxygen: P4 + 5O2 → P4O10 The crude product may contain a small amount of sesquioxide, P2O3, which may be removed by sublimation in ozonized oxygen. |
| Production Methods | Phosphorus pentoxide or phosphoric anhydride (P2O5) is formed by burning yellow phosphorus in dry air or oxygen. |
| Definition | ChEBI: Diphosphonate(2-) is a divalent inorganic anion obtained by removal of both protons from diphosphonic acid. It is a phosphorus oxoanion and a divalent inorganic anion. It is a conjugate base of a diphosphonate(1-). |
| General Description | A white amorphous powder. Corrosive to metals and tissue and moderately toxic. |
| Air & Water Reactions | Readily absorbs moisture from the air forming a syrup of meta-, pyro-, and orthophosphoric acids. Reacts violently with water releasing considerable heat [Oldbury Chemicals, p. 9]. |
| Reactivity Profile | Phosphorus pentoxide reacts violently and exothermically with water. The heat can ignite surrounding or admixed combustible materials. Undergoes hazardous or violent reactions with metal hydroxides and oxides, formic acid, hydrogen fluoride and hydrofluoric acid, iodides, metals (in particular potassium and sodium), oxidizing agents (bromine pentafluoride, chlorine trifluoride, perchloric acid, oxygen difluoride, hydrogen peroxide), ammonia, and proparygl alcohol. [Bretherick, 5th ed., 1995, p. 1781; EPA, 1998]. A violent explosion occurs if a solution of perchloric acid in chloroform is poured over phosphorus pentaoxide [EPA, 1998]. |
| Hazard | Phosphorus pentoxide is a strong irritant. It is corrosive to skin and contact with eyes can be injurious. |
| Health Hazard | Because of its dehydrating action, phosphorus pentoxide is a highly corrosive substance. It is an irritant to the eyes, skin, and mucous membranes. Inhalation of its vapors caused chronic pulmonary edema, injury to lungs, and hemorrhage in test animals. The LC50 values varied significantly with the species. LC50 value, inhalation (rats): 127 mg/m3/h . |
| Fire Hazard | Reacts violently with water to evolve heat. Flammable poisonous gases may accumulate in tanks and hopper cars. Phosphorus pentoxide reacts violently with the following: ammonia, hydrofluoric acid, oxygen difluoride, potassium, sodium, propargyl alcohol, calcium oxide, sodium hydroxide and chlorine trifluoride. A violent explosion occurs if a solution of perchloric acid in chloroform is poured over phosphorus pentoxide. Avoid formic acid, hydrogen fluoride, inorganic bases, metals, oxidants, water. Readily absorbs moisture from air to form meta-, pryo-, or orthophosphoric acid. |
| Agricultural Uses | Phosphoric anhydride is another name for phosphorus pentoxide (P2O5). Anhydrides react with water to give acids. For example, sulphur trioxide reacts with water to give sulphuric acid, or acetic anhydride reacts with water to give acetic acid. Similarly, phosphorus pentoxide has great affinity for water and dissolves in it to give phosphoric acid, and therefore, is known as phosphoric anhydride. |
| Safety Profile | Poison by inhalation. A corrosive irritant to the eyes, shin, and mucous membranes. With the appropriate conditions it undergoes hazardous reactions with formic acid, hydrogen fluoride, inorganic bases, iodides, metals, methyl hydroperoxide, oxidants (e.g., bromine, pentafluoride, chlorine trifluoride, perchloric acid, oxygen Difluoride, hydrogen peroxide), 3-propynol, water. When heated to decomposition it emits toxic fumes of POx. |
| Potential Exposure | This material is used as an intermediate in organic synthesis, catalyst, condensing agent; dehydrating agent; in the preparation of acrylate esters, surfactants, sugar refining; medicine, fire extinguishing; and special glasses. |
| Purification Methods | It has been sublimed at 250o under vacuum into glass ampoules. It fumes in moist air and reacts violently with water. It is an excellent drying agent for use in desiccators. HARMFUL VAPOURS and attacks skin. [Manley J Chem Soc 121 331 1922, Klements in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 541 1963.] |
| Incompatibilities | Reacts violently and exothermically with water, forming ignition level heat and highly corrosive phosphoric acid. Keep away from the combination of moisture and combustible materials. Phosphorus pentoxide reacts violently with the following: perchloric acid; ammonia, hydrofluoric acid; oxidizers, hydrogen fluoride; formic acid, oxygen difluoride, potassium, sodium, propargyl alcohol; calcium oxide; inorganic bases; sodium hydroxide and chlorine trifluoride. Attacks many metals in presence of water. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, alcohols, ammonia. Undergoes hazardous or violent reactions with metal hydroxides and oxides, formic acid, hydrogen fluoride and hydrofluoric acid, iodides, metals (in particular potassium and sodium), ammonia, and proparygl alcohol. |
| Waste Disposal | Decompose with water, forming phosphoric and hydrochloric acids. The acids may then be neutralized and diluted slowly to solution of soda ash and slaked lime with stirring then flush to sewer with large volumes of water. |
| Phosphorus pentoxide Preparation Products And Raw materials |