| NICKEL(II) BROMIDE TRIHYDRATE Basic information |
| Product Name: | NICKEL(II) BROMIDE TRIHYDRATE |
| Synonyms: | NICKEL BROMIDE;NICKEL(+2)BROMIDE;NICKEL(II) BROMIDE;NICKEL(II) BROMIDE-3-HYDRATE;NICKEL(II) BROMIDE TRIHYDRATE;NICKEL BROMIDE (OUS);NICKEL BROMIDE TRIHYDRATE;NICKELOUS BROMIDE |
| CAS: | 7789-49-3 |
| MF: | Br2H2NiO |
| MW: | 236.52 |
| EINECS: | 677-463-6 |
| Product Categories: | Catalysis and Inorganic Chemistry;Chemical Synthesis;Nickel Salts;NickelMetal and Ceramic Science;Salts |
| Mol File: | 7789-49-3.mol |
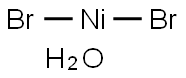 |
| NICKEL(II) BROMIDE TRIHYDRATE Chemical Properties |
| Melting point | 963 °C(lit.) |
| density | 5.098 g/mL at 25 °C(lit.) |
| form | powder |
| Water Solubility | Soluble in water, alcohol |
| Sensitive | Hygroscopic |
| Merck | 14,6502 |
| Exposure limits | ACGIH: TWA 0.1 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 |
| CAS DataBase Reference | 7789-49-3(CAS DataBase Reference) |
| Safety Information |
| Hazard Codes | T,N |
| Risk Statements | 45-22-43-50/53 |
| Safety Statements | 53-36/37-45-60-61 |
| RIDADR | UN 3077 9/PG 3 |
| WGK Germany | 3 |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | III |
| Provider | Language |
|---|---|
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
| NICKEL(II) BROMIDE TRIHYDRATE Usage And Synthesis |
| Chemical Properties | The green hexahydrate NiBr2-6H2O readily evolves water; crystallization from aqueous solutions above 29° yields the trihydrate NiBr2-3H2O. The hexahydrate can be dehydrated to a dihydrate over concentrated sulphuric acid at 5°. The environment of the nickel atoms in NiBr2-6H20 is 2Br and 4H20 and in NiBr2-2H20 4Br and 2H20 ; in both cases the Ni-Br distance is 2.6 and the Ni-O distance 2.0 ?. |
| Chemical Properties | (1) Brownish-yellow solid or yellow, lustrous scales.(2) Deliquescent, greenish scales; loses 3H2O at 300C. Soluble in water, alcohol, ether, and ammonium hydroxide. |
| Uses | It is used in Soporific and sedatives pharmaceutical Industry, Photosensitive industry, Analytical Chemistry, and also electrolyte in high energy battery. |
| Preparation | Anhydrous NiBr2 is most conveniently prepared by the action of bromine on nickel, either at red heat or in ethereal solution at room temperature74 or by dehydration of the hexahydrate at about 140°. It crystallizes with the CdCl2 type structure, the nickel atoms being surrounded octahedrally by bromine atoms at 2.57 ?. |
| Hazard | See nickel. |