| Hydrogen bromide Basic information |
| Chemical properties Uses production method Hazards & Safety Information |
| Product Name: | Hydrogen bromide |
| Synonyms: | Acide bromhydrique;acidebromhydrique;acidebromhydrique(french);Acido bromidrico;acidobromhidrico;acidobromidrico;Anhydrous hydrobromic acid;anhydroushydrobromicacid |
| CAS: | 10035-10-6 |
| MF: | BrH |
| MW: | 80.91 |
| EINECS: | 233-113-0 |
| Product Categories: | Liqiud crystal intermediates;Acids&Bases;Digestion Reagents;Essential Chemicals;Ultrapure Reagents for Wet Digestion (Trace SELECTUltra);Chemical Synthesis;inorganic acid;Biochemistry;Reagents for Oligosaccharide Synthesis;ACS GradeChemical Synthesis;Analytical Reagents for General Use;E-L, Puriss p.a. ACS;Puriss p.a. ACS;Halogenated SolutionsChemical Synthesis;Acid SolutionsTitration;Inorganic Acids;Synthetic Reagents;Titration;Volumetric Solutions;TraceSelectUltraAtomic Absorption Spectroscopy (AAS);Inorganics;10035-10-6 |
| Mol File: | 10035-10-6.mol |
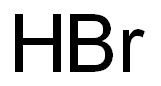 |
| Hydrogen bromide Chemical Properties |
| Melting point | −87 °C(lit.) |
| Boiling point | −67 °C(lit.) |
| density | 1.49 g/mL at 25 °C (lit.) |
| vapor density | 2.8 (vs air) |
| vapor pressure | 334.7 psi ( 21 °C) |
| refractive index | n20/D 1.438 |
| Fp | 40°C |
| storage temp. | Store below +30°C. |
| solubility | soluble |
| pka | -9(at 25℃) |
| form | Solution |
| Specific Gravity | 1.49 |
| color | Light yellow, brown |
| Odor | Sharp, irritating odor detectable at 2 ppm |
| PH | 3.01(1 mM solution);2.04(10 mM solution);1.08(100 mM solution) |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,4778 |
| BRN | 3587158 |
| Exposure limits | Ceiling limit 3 ppm (~10 mg/m3) (ACGIH); TLV-TWA 3 ppm (~10 mg/m3) (MSHA and OSHA). |
| Stability: | Stable. Incompatible with strong bases, strong oxidizing agents, ammonia, ozone, fluorine, water, metals. Air and light sensitive. |
| LogP | 0.629 at 25℃ |
| CAS DataBase Reference | 10035-10-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Hydrogen bromide(10035-10-6) |
| EPA Substance Registry System | Hydrobromic acid (10035-10-6) |
| Safety Information |
| Hazard Codes | C,Xi |
| Risk Statements | 35-37-34-10-36/37/38 |
| Safety Statements | 26-45-7/9-36/37/39 |
| RIDADR | UN 3265 8/PG 2 |
| WGK Germany | 1 |
| RTECS | MW3850000 |
| TSCA | Yes |
| DOT Classification | 2.3, Hazard Zone C (Gas poisonous by inhalation) |
| HazardClass | 8 |
| PackingGroup | II |
| HS Code | 28111990 |
| Hazardous Substances Data | 10035-10-6(Hazardous Substances Data) |
| Toxicity | LC50 in mice, rats: 814, 2858 ppm by inhalation, K. C. Back et al., Reclassification of Materials Listed as Transportation Health Hazards (TSA-20-72-3, PB 214-270, 1972) |
| IDLA | 30 ppm |
| Provider | Language |
|---|---|
| Hydrogen bromide | English |
| SigmaAldrich | English |
| ACROS | English |
| ALFA | English |
| Hydrogen bromide Usage And Synthesis |
| Chemical properties | Colorless or light yellow liquid, slightly smoke. Soluble in chlorobenzene, diethoxymethane and other organic solvents. Can be miscibled with water, alcohol, acetic acid. |
| Uses | (1) Hydrogen bromide is the basic raw material for the manufacture of a variety of inorganic bromide (Such as sodium bromide, potassium bromide, lithium bromide and calcium bromide and so on) and some alkyl bromide (Such as methyl bromide, bromoethane and so on). It is used in medicine to synthesize the synthesis of sedatives and anesthetics, etc. and also is a good solvent for some metal minerals, used in the refinement of high purity metals. In the petroleum industry, it is used as the separation of alkoxy and phenoxy compounds, and a catalyst for the oxidation of cyclic hydrocarbons and chain hydrocarbons to ketones, acid or peroxide. Also used in synthetic dyes and spices. (2) For the manufacture of inorganic and organic bromide; also used for synthetic perfumes, dyes and so on. (3) For the refinement of high purity and bromide synthesis, also used as analytical reagents (4) Determination of sulfur, selenium, bismuth, zinc and iron. Separation of tin from arsenic and antimony. Alkylation catalyst. Reducing agent. Organic Synthesis. Preparation of organic and inorganic bromides. High purity metal refining. (5) It is the basic raw material for the manufacture of a variety of inorganic bromide (Such as sodium bromide, potassium bromide, lithium bromide and calcium bromide and so on ) and some alkyl bromide (Such as methyl bromide, bromoethane and so on). It is used in medicine to synthesize the synthesis of sedatives and anesthetics, etc. and also is a good solvent for some metal minerals, used in the refinement of high purity metals. In the petroleum industry, it is used as the separation of alkoxy and phenoxy compounds, and a catalyst for the oxidation of cyclic hydrocarbons and chain hydrocarbons to ketones, acid or peroxide. Also used in synthetic dyes and spices. It is used in the manufacture of various bromine compounds, can also be used for medicine, dyes, spices and other industries. For the purification of high purity and synthesis of bromide, and also for analysis reagents. For the manufacture of inorganic and organic bromide; also used for synthetic perfumes, dyes and so on. (6) Used as analytical reagent. Determination of sulfur and selenium, separation of tin from arsenic and antimony, determination of bismuth, zinc and iron, alkylation catalyst. |
| production method | (1) Bromine and hydrogen reaction of hydrogen bromide in the Presence of Activated Carbon Catalyst. Followed by distillation and purification to obtain hydrogen bromide. Br2+H2→2HBr (2) Red phosphorus method: First, the red phosphorus into the water reactor, slowly adding bromine under stirring, the reaction of hydrobromic acid and phosphoric acid, by sedimentation, filtration, distillation obtained hydrobromic acid. P4+6Br2+12H2O→12HBr+4H3PO3 Sulfur dioxide method: The sulfur dioxide is added into the reaction kettle with bromine and crushed ice, and the reaction is continued until the temperature is below 20 ℃ until the solution is yellow. The mixed solution is distilled, and the solution is added to the solution of barium hydroxide and sulfuric acid to produce barium sulfate precipitation. After standing, filtering, remove the precipitate, then distillation of the filtrate was hydrobromic acid products. |
| Hazards & Safety Information | Category: Harmful gas Toxicity classification: Poisoning Acute toxicity: Inhalation-Rats LC50: 2858 PPM/h; Inhalation-mice LC50: 814 PPM/h Hazardous properties of explosives: Mixed with air explosion Flammability hazard characteristics: It is combustible in case of H hair pore agent; Case of cyanide release of toxic hydrogen cyanide gas; Thermal decomposition of toxic bromide gas. Storage and transportation characteristics: Low temperature and dry storage; and cyanide, Separate storage with cyanide, H hair pore agent, and alkali. Fire extinguishing agent: water Professional standards: TWA 3 PPM (10mg/m3) |
| Description | Hydrobromic Acid is a strong acid formed by dissolving the diatomic molecule HBr in water. “Constant-boiling” hydrobromic acid is an aqueous solution that distills at 124.3°C and contains 47.6% HBr by weight. Hydrobromic acid has a pKa of 9, making it a stronger acid than hydrochloric acid, but not as strong as HI, hydroiodic acid. Hydrobromic acid is one of the strongest mineral acids known. Hydrobromic acid is mainly used for the production of inorganic bromides, especially the bromides of zinc, calcium, and sodium. It is a useful reagent for generating organobromine compounds. Certain ethers are cleaved with HBr. It also catalyzes alkylation reactions and the extraction of certain ores. Industrially significant organic compounds prepared from hydrobromic acid include allyl bromide, tetrabromobis(phenol), and bromoacetic acid. Hydrobromic acid can be prepared in the laboratory via the reaction of Br2, SO2 and water. Another laboratory preparation involves the production of anhydrous HBr, which is then dissolved in water. Hydrobromic acid has commonly been prepared industrially by reacting bromine with either sulfur or phosphorous and water. However, it can also be produced electrolytically. It can also be prepared by treating bromides with nonoxidizing acids like phosphoric or acetic acids. Hydrobromic acid is available commercially in various concentrations and purities. |
| Chemical Properties | colourless liquid with a strong irritating odour |
| Physical properties | Colorless gas; fumes in moist air; pungent acrid odor; nonflammable; heav-ier than air; density 2.71 (air=1.0); gas density 3.55 g/L at 25°C; liquefies at-66.4°C; solidifies at -86.8°C; critical temperature 89.8°C; critical pressure84.5 atm; highly soluble in water (saturated aqueous solution contains 66%HBr at 25°C); forms a constant-boiling azeotrope at 47.5% HBr in solution,boiling at 126°C at atmospheric pressure; soluble in alcohol; a 0.10Maqueoussolution is 93% ionized to H+and Br ? ions at 18°C. |
| Uses | Hydrobromic acid is used in the manufacture of bromide, as an alkylation catalyst, and in organic synthesis. |
| Uses | Hydrogen bromide is used as a reagent and catalyst in several types of organic reactions such as the formation of alkyl bromides from alcohols. It is also used as a source material in the preparation of inorganic bromides. Hydrogen bromide serves as a catalyst in alkylation reactions. It has also been reportedly used in the controlled oxidation of aliphatic and alicyclic hydrocarbons to peroxides, ketones, and acids. In organic synthesis, hydrogen bromide is used to substitute bromine for aliphatic chlorine in the presence of aluminum catalyst. |
| Uses | The Concentrated acid is used principally in analytical chemistry and organic preparations. |
| Definition | A colorless liquid produced by adding hydrogen bromide to water. It shows the typical properties of a strong acid and it is a strong reducing agent. A convenient way of producing hydrobromic acid is to bubble hydrogen sulfide through bromine water. Although it is not as strong as hydrochloric acid it dissociates extensively in water and is a good proton donor. |
| Definition | Hydrogen bromide in aqueous solution. |
| Preparation | Hydrogen bromide gas may be produced by combustion of hydrogen inbromine vapor at 37.5°C using a catalyst such as platinized asbestos or pla-tinized silica gel. Unreacted free bromine is removed from the product bypassing the gaseous product mixture over hot activated charcoal. Hydrogenbromide formed may be absorbed in water to obtain the acid; or may be cooledand liquefied for shipment in cylinders. Hydrobromic acid may be prepared in the laboratory by distillation of asolution of potassium bromide with dilute sulfuric acid: 2KBr + H2SO4 → K2SO4 + HBr The acid may be prepared by several other methods, as well, including reac-tion of bromine either with sulfur and water; or with phosphorus and water: 2Br2 + S + 2H2O → 4HBr + SO2 Hydrobromic acid also may be prepared by hydrogen exchange with a sodiumor potassium bromide solution when the solution is passed through a cation-exchange resin. Hydrobromic acid is stored and shipped in drums, tanks, carboys, or bot-tles, labeled as corrosive materials. The anhydrous gas is stored and shippedin cylinders under its vapor pressure. |
| Definition | hydrogen bromide: A colourlessgas, HBr; m.p. –88.5°C; b.p. –67°C. Itcan be made by direct combinationof the elements using a platinum catalyst.It is a strong acid dissociatingextensively in solution (hydrobromicacid). |
| General Description | Hydrobromic acid solution (HBr) is a clear, yellow or brown colored liquid. Its reaction with K has been studied by a molecular beam technique. |
| Air & Water Reactions | Acrid odor, fumes in moist air forming clouds containing hydrobromic acid. Heat of solution large, [Merck, 11th ed., 1989]. |
| Reactivity Profile | HYDROGEN BROMIDE is an anhydrous (no water) strong acid. Reacts rapidly and exothermically with bases of all kinds (including amines and amides). Reacts exothermically with carbonates (including limestone and building materials containing limestone) and hydrogen carbonates to generate carbon dioxide. Reacts with sulfides, carbides, borides, and phosphides to generate toxic or flammable gases. Reacts with many metals (including aluminum, zinc, calcium, magnesium, iron, tin and all of the alkali metals) to generate flammable hydrogen gas. Reacts violently with acetic anhydride, 2-aminoethanol, ammonium hydroxide, calcium phosphide, chlorosulfonic acid, 1,1-difluoroethylene, ethylenediamine, ethyleneimine, oleum, perchloric acid, b-propiolactone, propylene oxide, silver perchlorate/carbon tetrachloride mixture, sodium hydroxide, uranium(IV) phosphide, vinyl acetate, calcium carbide, rubidium carbide, cesium acetylide, rubidium acetylide, magnesium boride, mercury(II) sulfate, calcium phosphide, calcium carbide. |
| Hazard | Toxic by inhalation, strong irritant to eyes and skin. |
| Health Hazard | Hydrobromic acid is a corrosive liquid. Thegas is a strong irritant to the eyes, nose, andmucous membranes. In humans, exposure to5 ppm for a few minutes can cause irritationof the nose. Irritation of the eyes and lungsmay be felt at higher concentrations. Thedetectable odor threshold is 2 ppm. |
| Health Hazard | Hydrobromic acid and hydrogen bromide gas are highly corrosive substances that can cause severe burns upon contact with all body tissues. The aqueous acid and gas are strong eye irritants and lacrimators. Contact of concentrated hydrobromic acid or concentrated HBr vapor with the eyes may cause severe injury, resulting in permanent impairment of vision and possible blindness. Skin contact with the acid or HBr gas can produce severe burns. Ingestion can lead to severe burns of the mouth, throat, and gastrointestinal system and can be fatal. Inhalation of hydrogen bromide gas can cause extreme irritation and injury to the upper respiratory tract and lungs, and exposure to high concentrations may cause death. HBr gas is regarded as having adequate warning properties. Hydrogen bromide has not been found to be carcinogenic or to show reproductive or developmental toxicity in humans. |
| Fire Hazard | Behavior in Fire: Pressurized container may explode and release toxic, irritating vapor. |
| Flammability and Explosibility | Noncombustible, but contact with metals may produce highly flammable hydrogen gas. |
| Materials Uses | Hydrogen bromide does not aggressively attack common metals of construction while in the anhydrous state. However, in the presence of moisture, hydrogen bromide will attack most metals except platinum and silver. Galvanized pipe, brass, and bronze should be avoided. Steel, Monel, and aluminum-silicon-bronze have proven satisfactory in anhydrous hydrogen bromide service. |
| Physiological effects | Hydrogen bromide is extremely irritating to the eyes, mucous membranes of the respiratory tract, and skin. Contact may cause bums. Repeated short exposures to concentrations of about 35 ppm can cause irritation to the nose and throat with mucous production and indigestion. Inhalation of higher concentrations can cause pulmonary edema and laryngeal spasm, and can be fatal. Skin contact with the vapor or liquid causes severe tissue irritation and necrosis [2]. |
| storage | Splash goggles and rubber gloves should be worn when handling this acid, and containers of HBr should be stored in a wellventilated location separated from incompatible metals. Water should never be added to HBr because splattering may result; always add acid to water. Containers of hydrobromic acid should be stored in secondary plastic trays to avoid corrosion of metal storage shelves due to drips or spills. Cylinders of hydrogen bromide should be stored in cool, dry locations, separated from alkali metals and other incompatible substances. |
| Purification Methods | A solution of aqueous HBr ca 48% (w/w, constant boiling) is purified by distilling twice with a little red phosphorus, and the middle half of the distillate is taken. (The azeotrope at 760mm contains 47.8% (w/w) HBr.) [Hetzer et al. J Phys Chem 66 1423 1962]. Free bromine can be removed by Irvine and Wilson’s method for HI (see above), except that the column is regenerated by washing with an ethanolic solution of aniline or styrene. Hydrobromic acid can also be purified by aerating with H2S, distilling and collecting the fraction boiling at 125-127o. [Heisig & Andur Inorg Synth I 155 1939.] HARMFUL VAPOURS. |
| Incompatibilities | Hydrobromic acid and hydrogen bromide react violently with many metals with the generation of highly flammable hydrogen gas, which may explode. Reaction with oxidizers such as permanganates, chlorates, chlorites, and hypochlorites may produce chlorine or bromine. |
| Waste Disposal | In many localities, hydrobromic acid or the residue from a spill may be disposed of down the drain after appropriate dilution and neutralization. Otherwise, hydrobromic acid and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution’s waste disposal guidelines. Excess hydrogen bromide in cylinders should be returned to the manufacturer. For more information on disposal procedures, see Chapter 7 of this volume. |
| GRADES AVAILABLE | Hydrogen bromide is typically available in 99.8 percent purity. Gas purity guidelines have been developed and published by Semiconductor Equipment and Materials International and can be found in the Book of SEMI Standards, Gases Volume. |
| Hydrogen bromide Preparation Products And Raw materials |