| Cobalt Basic information |
| Chemical Properties Physical properties History Uses Production Methods |
| Product Name: | Cobalt |
| Synonyms: | Cobalt, plasma standard solution, Co 10μg/mL;Cobalt wire, 0.1mm (0.004 in.) dia.;Cobalt wire, 0.25mm (0.01 in.) dia.;Cobalt slug, 3.175mm (0.125 in.) dia. x 3.175mm (0.125 in.) length;Cobalt rod, 12mm (0.47 in.) dia.;Cobalt sputtering target, 50.8mm (2.0 in.) dia. x 3.18mm (0.125 in.) thick;Cobalt sputtering target, 76.2mm (3.0 in.) dia. x 6.35mm (0.250 in.) thick;Cobalt foil, 0.127mm (0.005 in.) thick, 50x50mm (2×2 in.) |
| CAS: | 7440-48-4 |
| MF: | Co |
| MW: | 58.93 |
| EINECS: | 231-158-0 |
| Product Categories: | metal or element;nano structured metal;Metals;Inorganics;Catalysis and Inorganic Chemistry;Chemical Synthesis;CobaltMetal and Ceramic Science;Cobalt;Metal and Ceramic Science;C-D, Puriss p.a.Chemical Synthesis;Analytical Reagents for General Use;Puriss p.a. |
| Mol File: | 7440-48-4.mol |
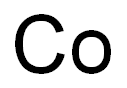 |
| Cobalt Chemical Properties |
| Melting point | 1495°C |
| Boiling point | 2900 °C (lit.) |
| density | 8.9 g/mL at 25 °C (lit.) |
| vapor pressure | 0Pa at 20℃ |
| storage temp. | no restrictions. |
| solubility | H2O: soluble |
| form | wire |
| Specific Gravity | 8.9 |
| color | Pink to red to violet |
| resistivity | 6.24 μΩ-cm, 20°C |
| Water Solubility | insoluble |
| Merck | 13,2452 |
| Exposure limits | TLV-TWA 0.05 mg as Co/m3 (ACGIH) PEL-TWA: 0.05 mg as Co/m3 (NIOSH, OSHA) TLV-STEL 0.1 mg as Co/m3 (ACGIH) IDLH 20 mg as Co/m3 (NIOSH) . |
| Stability: | Stable, but pyrophoric in air when finely divided. Incompatible with acetylene, hydrazinium nitrate, oxidizing agents, acids. |
| CAS DataBase Reference | 7440-48-4(CAS DataBase Reference) |
| IARC | 2B (Vol. 52) 1991, 2B (Vol. 86) 2006, 2A (Vol. 86) 2006 |
| NIST Chemistry Reference | Cobalt(7440-48-4) |
| EPA Substance Registry System | Cobalt (7440-48-4) |
| Safety Information |
| Hazard Codes | T,Xn,F |
| Risk Statements | 45-23/24/25-34-53-42/43-40-36/37-36/38-17-11-15 |
| Safety Statements | 53-23-26-36/37/39-45-61-37-24-22-36/37-5-43 |
| RIDADR | UN 3264 8/PG 3 |
| WGK Germany | 3 |
| RTECS | GF8750000 |
| TSCA | Yes |
| HS Code | 8105 20 00 |
| HazardClass | 4.1 |
| PackingGroup | III |
| Hazardous Substances Data | 7440-48-4(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 6170 mg/kg |
| IDLA | 20 mg Co/m3 |
| Provider | Language |
|---|---|
| ACROS | English |
| SigmaAldrich | English |
| ALFA | English |
| Cobalt Usage And Synthesis |
| Chemical Properties | Cobalt is a silvery-gray brittle but hard metal, which is distributed widely in nature, including rocks, soil, plants, and animals. It is a metal with no odor and no vapor pressure at room temperature and is insoluble in water, except for ultrafine cobalt powder, which is water soluble. It is stable in air but chemically reactive with dilute acids and is a nonvolatile metal. Required in small amounts, cobalt is important for the sustenance of life. It is the only metal found in vitamins, most importantly as a part of vitamin B12 and is necessary in the formation of blood. Cobalt is a component of many naturally occurring minerals like sulfides, hydrates, and oxides. Cobalt compounds can occur in various oxidation states (0, +1, +2, +3, and +4) but the most common oxidation states are +2 and +3. Cobalt has one naturally occurring isotope, cobalt-59 and 26 radioactive isotopes. Cobalt and its compounds are used as an important catalyst in various industrial processes. Cobalt catalysts are used in various processes such as a desulfurization catalyst for gas and oil and for polyesters, pottery, plastic, and detergent production. Cobalt is usually recovered as a byproduct of mining and refining of various minerals like copper and iron. It has the unique ability to be ductile as well as a malleable metal. This property allows it to be transformed into both thin wires and thin sheets. |
| Physical properties | Cobalt is a hard magnetic metal, like iron and nickel. It has the properties of both ductility as well as malleability. Due to this property, it can be molded into wires and compressed into sheets. Cobalt occurs in two allotropic forms, hexagonal and cubic. The hexagonal form is more stable than the cubic form at room temperature. The boiling point of cobalt is about 2927 °C (5301°F) and the melting point is about 1490 °C (2723°F). Cobalt as a metal is mildly reactive with oxygen and will burn only in powder form. It is soluble in dilute acids but is water soluble only in ultrafine powder form (Kyono et al., 1992). |
| History | The metal was isolated by Brandt in 1735 and confirmed as an element by Bergman in 1780. Cobalt is widely distributed in nature, but in small concentrations. Its concentration in the earth’s crust is estimated to be about 0.0025% and in the sea water is about 0.02 µg/L. Most cobalt found on earth is diffused into the rocks. It also is found in coal and soils, and at trace concentations in animals and plants. It is an essential element for plants and animals (as vitamin B12). Its absence in animals can cause retarded growth, anemia and loss of apetite. The element has been detected in meteorites and in the atmospheres of the sun and other stars. |
| Uses | Cobalt has been in use as a coloring agent for glass since ancient times. The most imporant use of cobalt is in the manufacture of various wearresistant and superalloys. Its alloys have shown high resistance to corrosion and oxidation at high temperatures. They are used in machine components. Also, certain alloys are used in desulfurization and liquefaction of coal and hydrocracking of crude oil shale. Cobalt catalysts are used in many industrial processes. Several cobalt salts have wide commercial applications Cobalt oxide is used in glass to impart pink or blue color. Radioactive cobalt–60 is used in radiography and sterilization of food. |
| Production Methods | Cobalt is obtained from its ores, which are mostly sulfide, arsenic sulfide or oxide in nature. The finely ground ore is subjected to multistep processing, depending on the chemical nature of the ore. When the sulfide ore carrollite, CuS•Co2S3, is the starting material, first sulfides are separated by flotation with frothers. Various flotation processes are applied. The products are then treated with dilute sulfuric acid producing a solution known as copper-cobalt concentrate. This solution is then electrolyzed to remove copper. After the removal of copper, the solution is treated with calcium hydroxide to precipitate cobalt as hydroxide. Cobalt hydroxide is filtered out and separated from other impurities. Pure cobalt hydroxide then is dissolved in sulfuric acid and the solution is again electrolyzed. Electrolysis deposits metallic cobalt on the cathode. Production of cobalt in general is based on various physical and chemical processes that include magnetic separation (for arsenic sufide ores), sulfatizing roasting (for sulfide ores), ammoniacal leaching, catalytic reduction, and electrolysis. Finely divided cobalt particles can be prepared by reduction of cobalt(II) chloride by lithium naphthalenide in glyme. |
| Description | Cobalt was discovered by George Brandt in 1737. Cobalt exists in valence states from 0 to 5, with the most stable (+2 and +3) being the most common. Although there is only one stable isotope of cobalt, there are a number of unstable isotopes. Two of these, cobalt-60 and cobalt-57, are in use commercially. Cobalt-60 is used for cancer treatment and food irradiation. Cobalt-57 has research applications. |
| Chemical Properties | There are two allotropic modifications of cobalt, a close-packed hexagonal form (ε) with space group P63/mmc, stable at temperatures below 417 °C; and a face-centered cubic form (α) with space group Fm3m, stable at higher temperatures—up to the melting point. The metal is silvery gray in color. The only naturally occurring isotope, 59Co, is stable, but the other twelve known isotopes are radioactive, their mass numbers ranging from 54 to 64. Half lives range from 0.2 second for 54Co to 5.3 years for the industrially and medically important 60Co. Cobalt was identified and described by Georg Brandt in 1735, but had to wait until the last decade of the nineteenth century before the new sources of metal supply from New Caledonia and Canada stimulated its metallurgical usage. |
| Chemical Properties | Cobalt is a silver-gray to black, hard, brittle, magnetic metal. It is relatively rare; the important mineral sources are the arsenides, sulfides, and oxidized forms. It is generally obtained as a by-product of other metals, particularly copper. The fume and dust of cobalt metal is odorless and black. The appearance and odor of cobalt compounds and their dusts and fumes vary with the compound. Cobalt metal in powdered form is incompatible with fused ammonium nitrate, hydrozinium nitrate, and strong oxidizing agents and should be avoided. It ignites on contact with bromide pentafl uoride. Powdered cobalt ignites spontaneously in air. Exposure to cobalt metal fume and dust can occur through inhalation, ingestion, and eye or skin contact. |
| Physical properties | Cobalt is a bluish steel-gray metal that can be polished to a bright shine. It is brittle andis not malleable unless alloyed with other metals. It is magnetic, and when alloyed with aluminum and nickel, it is called alnico metal, which acts as a super-magnet with many uses inindustry. Chemically and physically, cobalt acts much as do its two partners, iron (Fe) andnickel (Ni), located on each side of it in period 4 on the periodic table. In particular, iron,cobalt, and nickelare unique in that they possess natural magnetic properties. Cobalt’s meltingpoint is 1,495°C, its boiling point is 2,927°C, and its density is 8.86 g/cm3. |
| Isotopes | There are 33 isotopes of cobalt, ranging from Co-48 to Co-75, with half-livesranging from a few nanoseconds to 5.272 years for cobalt-60. Cobalt-59 is the onlystable isotope that constitutes almost all (roughly 100%) of the element’s natural presence on Earth. All the other isotopes are radioactive and are created artificially in nuclearreactors or nuclear explosions. |
| Origin of Name | Cobalt was given the name kobolds (or kolalds, or kololos) by German miners. It means “goblin” (see “History” for more on this story). |
| Occurrence | Cobalt is the 32nd most abundant element on Earth even though it makes up only 0.003%of the Earth’s crust. It is not found in the free metallic state, despite being widely distributedin igneous rocks as minerals. Its two most common mineral ores are cobaltite (CoAsS) anderythrite [Co3(AsO4)2]. These ores are placed in blast furnaces to produce cobalt arsenide(Co2As), which is then treated with sulfuric acid to remove the arsenic. Finally, the productcobalt tetraoxide (Co3O4) is reduced by heat with carbon (Co3O4 + C → 3Co + 2CO2), resulting in cobalt metal.Cobalt is also found in seawater, meteorites, and other ores such as linnaeite, chloanthite,and smaltite, and traces are found mixed with the ores of silver, copper, nickel, zinc, andmanganese. Cobalt ores are found in Canada and parts of Africa, but most of the cobalt usedin the United States is recovered as a by-product of the mining, smelting, and refining of theores of iron, nickel, lead, copper, and zinc. |
| Characteristics | Cobalt has the highest Curie point of any metal or alloy of cobalt. The Curie point is thetemperature at which an element will lose its magnetism before it reaches its melting point.Cobalt’s Curie point is 1,121°C, and its melting point is 1,495°C. About 25% of all cobaltmined in the world is used as an alloy with other metals. The most important is the alloyalnico, which consists of nickel, aluminum, and cobalt. Alnico is used to make powerful permanent magnets with many uses, such as CT, PET, and MRI medical instruments. It is alsoused for electroplating metals to give a fine surface that resists oxidation. |
| History | Cobalt occurs in the mineral cobaltite, smaltite, and erythrite, and is often associated with nickel, silver, lead, copper, and iron ores, from which it is most frequently obtained as a by-product. It is also present in meteorites. Important ore deposits are found in Congo-Kinshasa, Australia, Zambia, Russia, Canada, and elsewhere. The U.S. Geological Survey has announced that the bottom of the north central Pacific Ocean may have cobalt-rich deposits at relatively shallow depths in waters close to the Hawaiian Islands and other U.S. Pacific territories. Cobalt is a brittle, hard metal, closely resembling iron and nickel in appearance. It has a magnetic permeability of about two thirds that of iron. Cobalt tends to exist as a mixture of two allotropes over a wide temperature range; the β-form predominates below 400°C, and the α above that temperature. The transformation is sluggish and accounts in part for the wide variation in reported data on physical properties of cobalt. It is alloyed with iron, nickel and other metals to make Alnico, an alloy of unusual magnetic strength with many important uses. Stellite alloys, containing cobalt, chromium, and tungsten, are used for high-speed, heavy-duty, high-temperature cutting tools, and for dies. Cobalt is also used in other magnet steels and stainless steels, and in alloys used in jet turbines and gas turbine generators. The metal is used in electroplating because of its appearance, hardness, and resistance to oxidation. The salts have been used for centuries for the production of brilliant and permanent blue colors in porcelain, glass, pottery, tiles, and enamels. It is the principal ingredient in Sevre’s and Thenard’s blue. A solution of the chloride (CoCl2 · 6H2O) is used as sympathetic ink. The cobalt ammines are of interest; the oxide and the nitrate are important. Cobalt carefully used in the form of the chloride, sulfate, acetate, or nitrate has been found effective in correcting a certain mineral deficiency disease in animals. Soils should contain 0.13 to 0.30 ppm of cobalt for proper animal nutrition. Cobalt is found in Vitamin B-12, which is essential for human nutrition. Cobalt of 99.9+% purity is priced at about $250/kg. Cobalt-60, an artificial isotope, is an important gamma ray source, and is extensively used as a tracer and a radiotherapeutic agent. Single compact sources of Cobalt-60 vary from about $1 to $10/curie, depending on quantity and specific activity. Thirty isotopes and isomers of cobalt are known. |
| Uses | Cobalt is used in steel alloys, cementedcarbide abrasives and jet engines. |
| Uses | Cobalt has many practical uses.Historically, as well as today, different compounds of cobalt have been used for their colorsknown as cobalt blue, cerulean, new blue, smalt, cobalt yellow, and green.For many centuries cobalt was used to color glass, pottery, and porcelain and as an enamel.It is also used as a dye and paint pigment.As mentioned, cobalt alloyed with iron and nickel is used to make powerful permanentmagnets that are used in many industries.A major use is as an alloy with chromium to produce high-speed machine-cutting toolsthat are resistant to high temperatures.A cobalt alloy of copper and tungsten, called “stellite,” also maintains its hardness at hightemperatures, making it an ideal alloy for high-speed drills and cutting tools.The radioisotope cobalt-60, with a half-life of 5.27 years (1925.3 days) through beta (β)emission, decays to form the stable element nickel-60. It is used to test welds and metal castsfor flaws, to irradiate food crops to prolong freshness, as a portable source of ionizing gamma(γ) radiation, for radiation research, and for a medical source of radiation to treat cancers andother diseases.Cobalt is an important trace element for proper human nutrition. It is also a natural component of vitamin B12. |
| Uses | For alloys; manufacture of cobalt salts; in nuclear technology. Since 60Co can be encapsulated compactly, it has replaced radium in experimental medicine and cancer research. Cobalt is also used in the cobalt bomb, a hydrogen bomb surrounded by a cobalt metal shell. When the nuclear explosion occurs 60Co is formed from 59Co by neutron capture. Considered a “dirty bomb” because of long half-life and intense b- and g radiation. Max permissible concentration of 60Co in air: 10-7mCi/cc, Natl. Bur. Stand. Handb. 69, 31 (1959). |
| Definition | ChEBI: A cobalt group element atom that has atomic number 27. |
| Production Methods | World sources of the metal and the oxide are chiefly from Zaire, Belgium–Luxembourg, Norway, and Finland, in that order, with Zaire furnishing 58% of the world’s supply. Practically all cobalt produced is a by- or coproduct of other metals, chiefly copper; accordingly, a description of the mining process is omitted. The processes used in extracting cobalt from its ores vary according to the type of ore and locations of the ore deposit. Arsenical ores are concentrated by hand sorting, gravity separation, or froth flotation, and are smelted in a blast furnace with coke and limestone to a speiss (an impure mixture of iron, cobalt, and nickel arsenides). The speiss is ground, roasted with salt, and leached with water. Insoluble chlorides remaining after the leaching process are ground with sulfuric acid, washed, and filtered, and the washings are added to the liquid from the leaching step. The combined solution is oxidized and then neutralized with lime. Basic ferric arsenate precipitates and is removed, leaving a solution-containing cobalt and nickel. The addition of successive portions of sodium hydroxide and sodium hypochlorite precipitates cobalt as the hydroxide, which is initially pure but finally admixes with nickel hydroxide. The cobalt precipitate is dried, ground, and formed into pellets, which are reduced by heating with charcoal to cobalt metal. |
| Definition | A lustrous silveryblue hard ferromagnetic transition metal occurring in association with nickel. It is used in alloys for magnets, cutting tools, and electrical heating elements and in catalysts and some paints. |
| Definition | cobalt: Symbol Co. A light-greytransition element; a.n. 27; r.a.m.58.933; r.d. 8.9; m.p. 1495°C; b.p.2870°C. Cobalt is ferromagneticbelow its Curie point of 1150°C.Small amounts of metallic cobalt arepresent in meteorites but it is usuallyextracted from ore deposits workedin Canada, Morocco, and Za?re. It ispresent in the minerals cobaltite,smaltite, and erythrite but also associatedwith copper and nickel as sulphidesand arsenides. Cobalt ores areusually roasted to the oxide and thenreduced with carbon or water gas.Cobalt is usually alloyed for use. Alnicois a well-known magnetic alloyand cobalt is also used to make stainlesssteels and in high-strength alloysthat are resistant to oxidation at hightemperatures (for turbine blades andcutting tools). The metal is oxidized by hot airand also reacts with carbon, phosphorus,sulphur, and dilute mineralacids. Cobalt salts, usual oxidationstates II and III, are used to give abrilliant blue colour in glass, tiles,and pottery. Anhydrous cobalt(II)chloride paper is used as a qualitativetest for water and as a heat-sensitiveink. Small amounts of cobalt salts areessential in a balanced diet for mam-mals. Artificiallyproduced cobalt–60 is an importantradioactive tracer andcancer-treatment agent. The elementwas discovered by Georg Brandt(1694–1768) in 1737. |
| Reactions | Cobalt absorbs very little hydrogen even at high temperatures and nitrogen is practically insoluble up to 1200°C. Finely divided cobalt is pyrophoric in air, but the massive metal is scarcely attacked below 300°C. The oxide scale on cobalt heated in air or oxygen up to 900° consists of an outside layer of CO3O4 and a layer of CoO next to the metal ; above 900°, Co3O4 decomposes and the scale consists of CoO only. Cobalt reacts with many non-metals when heated, e.g. the halogens, boron, sulphur, phosphorus, arsenic and antimony, the reactions often proceeding with incandescence. Fluorine forms CoF3, while the other halogens give the cobalt(II) halide. |
| Brand name | C.i. 77320;Cobalt-59;Impromin;Inter-con;Kometileneamin;Levacide-c;Orkomin;Panacur;Sofracaps;Tasvite;Trelenium. |
| World Health Organization (WHO) | WHO Comment(non-radioactive forms): The World Health Organization has no information further to the above regarding preparations containing cobalt or to indicate that they are still commercially manufactured. |
| Air & Water Reactions | Burns brilliantly when exposed to air [Mellor 14:453(1946-1947)]. Insoluble in water. |
| Reactivity Profile | Pyrophoric Cobalt is a reducing agent. Decomposes acetylene in the cold as the metal becomes incandescent [Mellor 14:513(1946-1947]. Incompatible with oxidizing agents such as ammonium nitrate, bromine pentafluoride, and nitryl fluoride. |
| Hazard | Cobalt is found in most natural foods. Although a necessary trace element, it is toxic to humans if ingested in large amounts. The human body does excrete in urine excessive amounts of cobalt compounds such as found in vitamin B12. Cobaltous chromate (CoCrO4) is brownish-yellow to grayish-black (the color depends on its purity) is a dangerous carcinogen (causes cancer). Some years ago, a cobalt additive was used by some beer makers to maintain a foam head on their beer. Those who imbibed excessively developed what is known as beer drinkers syndrome, which caused some deaths from enlarged and flabby hearts. |
| Health Hazard | Cobalt is an essential element. Its deficiencycan result in pernicious anemia. It is present invitamin B12. Excessive intake of this elementmay result in polycythemia or overproductionof erythrocytes and heart lesions. Exposure toits dusts can produce cough and respiratoryirritation. Chronic inhalation of its dusts orfumes can decrease pulmonary functions andmay cause diffuse nodular fibrosis and otherpulmonary diseases. Skin contact may inducedermal hypersensitivity reactions, producingan allergy-type dermatitis. Co(II) ion is reported to be genotoxicin vitro and in vivo and carcinogenic inrodents (De Boeck et al. 2003) Occupationalexposure to hard metal (cemented carbide)dust is linked to an increased risk of lungcancer. |
| Fire Hazard | Literature sources indicate that the dust of Cobalt is flammable. |
| Flammability and Explosibility | Notclassified |
| Agricultural Uses | Cobalt (Co), a metallic element with an atomic weight of 58.94, is one of the transition elements belonging to the Group 9 (formerly Group VIII ) of the Periodic Table. However, in extremely low concentrations ranging from 0.1 to 10parts per billion (ppb), cobalt have been observed to improve growth, transpiration and photosynthesis of cotton, mustard and beans. Cobalt is required by symbiotic micro-organisms (e.g., rhizobia) for the fixation of elemental nitrogen through the formation of vitaminB12. Cobalt forms a complex with nitrogen atoms of the porphyrin ring structure and provides a prosthetic group for association with nucleotides in vitamin B12 co-enzyme. This complex is called cobamide. Other cobalt functions include leghemoglobin metabolism and ribonucleotide reductase in Rhizobium, and activation of enolase, lecithinase and succinic kinase. The concentration of cobalt in dry matter of plants ranges from 0.02 to 0.5 ppm. One ppb of cobalt in nutrient solution was found adequate for nitrogen fixation in alfalfa. The water content and catalase activity in leaves increased and the concentration of the cell sap decreased with cobalt application. Cobalt content in soil is low and variable. In India, for instance, it ranges from 4 to 80ppm. The humus content of the soil influences the availability of cobalt in it. The nature of clay affects the absorption of cobalt from solutions, in the order muscovite > hematite > bentonite = kaolin. An increase in the pH of the soil decreases the availability of cobalt. Cobalt deficiency is more pronounced in coarse sandy soils and under high rainfall conditions. To overcome deficiency, cobalt fertilization with 100to 200g/ha as cobaltous sulphate (CoSO,) is recommended. |
| Industrial uses | Cobalt (symbol Co) is a lustrous, silvery-bluemetallic chemical element, resembling nickelbut with a bluish tinge instead of the yellow ofnickel. It is rarer and costlier than nickel andits price has varied widely in recent years.Although allied to nickel, it has distinctive differences.It is more active chemically thannickel. It is dissolved by dilute H2SO4, HNO3,or HCl acids, and is attacked slowly by alkalis.The oxidation rate of pure cobalt is 25 timesthat of nickel. Its power of whitening copperalloys is inferior to that of nickel, but smallamounts in Ni–Cu alloys will neutralize theyellowish tinge of the nickel and make themwhiter. The metal is diamagnetic like nickel, buthas three times the maximum permeability.Like tungsten, it imparts red-hardness to toolsteels. It also hardens alloys to a greater extentthan nickel, especially in the presence of carbon,and can form more chemical compoundsin alloys than nickel. Its chemical properties resemble, in part,those of both nickel and iron. Cobalt is themetal with the highest Curie temperature(1121°C) and the lowest allotropic transformationtemperature (399°C). Below 421°C, cobaltis close-packed hexagonal; above, it is facecenteredcubic. |
| Biological Activity | Cobalt is a vital trace element in animal nutrition. Ruminants grazing upon cobaltdeficient pastures exhibit retarded growth, loss of appetite and anaemia ; rapid recovery from these symptoms occurs upon feeding the animals with a cobalt-supplemented diet. Cobalt salts are not therefore considered to be particularly toxic to animals, but to man they can in sufficiently large doses irritate the gastro-intestinal tract and cause nausea, vomiting and diarrhoea. Small amounts of cobalt, however, are invaluable in the treatment of pernicious anaemia. The discovery in 1926 of the antipernicious anaemia factor in liver led to the discovery in 1948 of vitamin B12, which was very soon after shown to contain cobalt. |
| Safety Profile | Confirmed carcinogen with experimental neoplastigenic and tumorigenic data. Poison by intravenous, intratracheal, and intraperitoneal routes. Moderately toxic by ingestion. Inhalation of the dust may cause pulmonary damage. The powder may cause dermatitis. Ingestion of soluble salts produces nausea and vomiting by local irritation. Powdered cobalt igmtes spontaneously in air. Flammable when exposed to heat or flame. Explosive reaction with hydrazinium nitrate, ammonium nitrate + heat, and 1,3,4,7-tetramethylisoindole (at 39OOC). Ignites on contact with bromine pentafluoride. Incandescent reaction with acetylene or nitryl fluoride. See also COBALT COMPOUNDS. |
| Potential Exposure | Possible risk of forming tumors, Suspected reprotoxic hazard. Nickel-aluminumcobalt alloys are used for permanent magnets. Alloys with nickel, aluminum, copper, beryllium, chromium, and molybdenum are used in the electrical, automobile, and aircraft industries. Cobalt is added to tool steels to improve their cutting qualities and is used as a binder in the manufacture of tungsten carbide tools. Various cobalt compounds are used as pigments in enamels, glazes, and paints; as catalysts in afterburners; and in the glass, pottery, photographic, electroplating industries. Radioactive cobalt (60Co) is used in the treatment of cancer. Cobalt has been added to beer to promote formation of foam but cobalt acts with alcohol to produce severe cardiac effects at concentrations as low as 1.2-1.5 mg/L of beer. Cobalt is part of the vitamin B12 molecule and as such is an essential nutrient. The requirement of humans for cobalt in the form of vitamin B12 is about 0.13 μg/day. |
| Carcinogenicity | In mammalian cells in vitro cobalt compounds have caused DNA strand breaks, sister chromatid exchanges, and aneuploidy, but not chromosomal aberrations.Cobalt salts are generally nonmutagenic in prokaryotic assays. |
| Environmental Fate | Cobalt most often depresses the activity of enzymes, including catalase, amino levulinic acid synthetase, and P-450, enzymes involved in cellular respiration. The Krebs citric acid cycle can be blocked by cobalt resulting in the inhibition of cellular energy production. Cobalt can replace zinc in a number of zincrequired enzymes such as alcohol dehydrogenase. Cobalt can also enhance the kinetics of some enzymes, such as heme oxidase in the liver. Cobalt interferes with and depresses iodine metabolism, resulting in reduced thyroid activity. Reduced thyroid activity can lead to goiter. |
| storage | Cobalt metal dust (powdered metal) should be stored in a cool, dry, well-ventilated area in tightly sealed containers that are labeled in accordance with OSHA standards. Containers of cobalt metal dust should be protected from physical damage and ignition sources, and should be stored separately from strong oxidizers. |
| Shipping | UN3189 Metal powder, self-heating, n.o.s., Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material |
| Toxicity evaluation | The sources of cobalt in the environment are both natural and synthetic (anthropogenic). Natural sources include soil, seawater spray, volcanic eruptions, and forest fires. Anthropogenic sources include combustion of fossil fuels, metal smelting, sewage sludge, and processing of cobalt alloys. Cobalt is found in the atmosphere in particulate form and returns to the Earth’s surface through dry deposition and with rain or snow. Once in surface water, cobalt generally moves into sediment. Cobalt does not appear to biomagnify significantly in the aquatic food chain. The cobalt that does accumulate in fish is largely found in the nonedible parts of the fish. Seventy-nine percent of the cobalt is transported by rivers globally and precipitates in estuaries. Under normal environmental conditions, cobalt is expected to bind strongly to soil; thus, migration through soil is very limited. Cobalt in soil can be taken up by plant roots and root vegetables, but does not translocate to the aboveground parts of plants. |
| Waste Disposal | Cobalt metal may be recovered from scrap and cobalt compounds from spent catalysts as alternatives to disposal. |
| Cobalt Preparation Products And Raw materials |