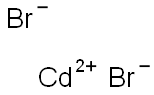| Chemical Properties | Cadmium bromide is a white to yellowish crystalline powder. |
| Chemical Properties | White to yellowish crysatlline powder |
| Physical properties | White to yellowish powder or flakes; hexagonal crystal system; hygroscopic; density 5.192g/cm3; melts at 568°C; vaporizes at 844°C; soluble in water, alcohol, ether, acetone, and liquid ammonia. |
| Uses | This compound is used in photography, engraving, and lithography. The other halogen elements also combine with cadmium in a similar ionic reaction as with bromine. |
| Uses | In photography, process engraving, and lithography. |
| Uses | Process engraving, lithography and photography |
| Uses | Made by heating cadmium to redness in bromine vapor. The yellowish crystalline powder is soluble in water and alcohol and is slightly soluble in ether. The crystals are deliquescent and must be kept in a well-stoppered bottle. Like its iodide counterpart, cadmium bromide was used in collodion in conjunction with an iodide of either ammonium or potassium. |
| Preparation | Cadmium bromide is prepared by heating cadmium with bromine vapor. Also the compound can be prepared by the treatment of dry cadmium acetate with glacial acetic acid and acetyl bromide. Alternatively, it may be obtained by dissolving cadmium or cadmium oxide in hydrobromic acid and evaporating the solution to dryness under helium in an inert atmosphere. |
| General Description | Odorless white solid. Mixes with water. |
| Air & Water Reactions | Water soluble. |
| Reactivity Profile | CADMIUM BROMIDE has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive. |
| Health Hazard | Inhalation causes coughing, sneezing, symptoms of lung damage. Ingestion produces severe toxic symptoms; both kidney and liver injuries may occur. Contact with eyes causes irritation. |
| Fire Hazard | Special Hazards of Combustion Products: Toxic cadmium oxide fumes may form in fires. |
| Safety Profile | Confirmed human carcinogen. When heated to decomposition it emits toxic fumes of Cd and Br-. |
| Potential Exposure | Cadmium bromide is used in photography, engraving, and lithography |
| Shipping | UN 2570 Cadmium compounds, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required |
| Purification Methods | Crystallise it from water (0.6mL/g) between 100o and 0o, and dry it at 110o. It forms the monohydrate below 36o and the tetrahydrate above 36o. [Wagenknecht & Juza in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1096 1965.] |