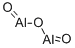| Chemical Properties | Aluminum is a combustible, light, silverywhite, soft, ductile, malleable, amphoteric metal |
| Uses | Depending on the formulator’s needs, aluminum oxide can be used as an abrasive, or for its absorbent, anti-caking, bulking, opacifying, or viscosity-controlling properties. |
| Definition | A white powder that is almost insoluble in water. Because of its amphoteric nature it will react with both acids and alkalis. Aluminum oxide occurs naturally as bauxite, corundum, and white sapphire; it is manufactured by heating aluminum hydroxide. It is used in the extraction by electrolysis of aluminum, as an abrasive (corundum), in furnace linings (because of its refractory properties), and as a catalyst (e.g. in the dehydration of alcohols). |
| Industrial uses | Alumina is the most widely used oxide,chiefly because it is plentiful, relatively lowin cost, and equal to or better than most oxidesin mechanical properties. Density can be variedover a wide range, as can purity — downto about 90% alumina — to meet specificapplication requirements. Alumina ceramicsare the hardest, strongest, and stiffest of theoxides. They are also outstanding in electricalresistivity, dielectric strength, are resistant toa wide variety of chemicals, and are unaffectedby air, water vapor, and sulfurous atmospheres.However, with a melting point ofonly 2039°C, they are relatively low in refractoriness,and at 1371°C retain only about 10%of room-temperature strength. In addition toits wide use as electrical insulators and itschemical and aerospace applications, the highhardness and close dimensional tolerancecapability of alumina make this ceramic suitablefor such abrasion-resistant parts as textileguides, pump plungers, chute linings, dischargeorifices, dies, and bearings. |
| Industrial uses | The oxide of aluminum is Al2O3. The naturalcrystalline mineral is called corundum, but thesynthetic crystals used for abrasives are designatedusually as aluminum oxide or marketedunder trade names. For other uses and as a powder it is generally called alumina. It iswidely distributed in nature in combinationwith silica and other minerals, and is an importantconstituent of the clays for making porcelain,bricks, pottery, and refractories.
The crushed and graded crystals of aluminawhen pure are nearly colorless, but the finepowder is white. Off colors are due to impurities.American aluminum oxide used for abrasivesis at least 99.5% pure, in nearly colorlesscrystals melting at 2050°C. The chief uses foralumina are for the production of aluminummetal and for abrasives, but it is also used forceramics, refractories, pigments, catalyst carriers,and in chemicals. |
| Potential Exposure | Most hazardous exposures to aluminum occur in smelting and refining processes. Aluminum is mostly produced by electrolysis of Al2O3 dissolved in molten cryolite (Na3AlF6). Aluminum is alloyed with copper, zinc, silicon, magnesium, manganese, and nickel; special additives may include chromium, lead, bismuth, titanium, zirconium, and vanadium. Aluminum and its alloys can be extruded or processed in rolling mills, wire works, forges, or foundries; and are used in the shipbuilding, electrical, building, aircraft, automobile, light engineering, and jewelry industries. Aluminum foil is widely used in packaging. Powdered aluminum is used in the paints and pyrotechnic industries. Alumina, emery, and corundum has been used for abrasives, refractories, and catalysts; and in the past in the first firing of china and pottery. |
| Shipping | UN1309 Aluminum powder, coated, Hazard Class: 4.1; Labels: 4.1-Flammable solid. UN1383 Pyrophoric metals, n.o.s. or Pyrophoric alloys, n.o.s., Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material, Technical Name Required. UN1396 Aluminum powder, uncoated, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material. NA9260 (North America) Aluminum, molten, Hazard class: 9; Labels: 9-Miscellaneous hazardous material. |
| Incompatibilities | Aluminum powder forms an explosive mixture with air and is a strong reducing agent that reacts violently with oxidizers, strong bases; strong acids; somehalogenated hydrocarbons; nitrates, sulfates, metal oxides and many other substances. Keep away from combustible materials. |
| Waste Disposal | Consult with environmental regulatory agencies for guidance on acceptable disposalpractices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal of Aluminum Oxide-Disposal in a sanitary landfill. Mixing of industrial process wastes and municipal wastes at such sites is not encouraged however. Aluminum powder may be recovered and sold as scrap. Recycling and recovery is a viable option to disposal for aluminum metal and aluminum fluoride (A-57). |